Predicting and Testing Bioavailability of Magnesium Supplements
- PMID: 31330811
- PMCID: PMC6683096
- DOI: 10.3390/nu11071663
Predicting and Testing Bioavailability of Magnesium Supplements
Abstract
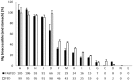
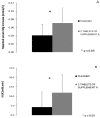
Despite the presumption of the beneficial effects of magnesium supplementation, little is known about the pharmacokinetics of different magnesium formulations. We aimed to investigate the value of two in vitro approaches to predict bioavailability of magnesium and to validate this in subsequent in vivo testing. In vitro assessment of 15 commercially available magnesium formulations was performed by means of a Simulator of the Human Intestinal Microbial Ecosystem (SHIME®) and by dissolution tests. Two magnesium formulations with contrasting bioavailability prediction from both in vitro tests (best vs. worst) were selected for in vivo testing in 30 subjects. In vivo bioavailability was compared following one acute ingestion by monitoring blood magnesium concentrations up to 6 h following intake. The in vitro tests showed a very wide variation in absorption and dissolution of the 15 magnesium products. In the in vivo testing, a significant different serum magnesium absorption profile was found up to 4 h following supplement ingestion for the two supplements with opposing in vitro test results. Moreover, maximal serum magnesium increase and total area under the curve were significantly different for both supplements (+6.2% vs. +4.6% and 6.87 vs. 0.31 mM.min, respectively). Collectively, poor bioaccessibility and bioavailability in the SHIME model clearly translated into poor dissolution and poor bioavailability in vivo. This provides a valid methodology for the prediction of in vivo bioavailability and effectiveness of micronutrients by specific in vitro approaches.
Keywords: Magnesium; bioaccessibility; bioavailability; supplements.
Conflict of interest statement
Oystershell Laboratories (Merelbeke, Belgium) provided the supplements used for in vitro and in vivo testing. Oystershell was not involved in the design of the study; in the collection, analyses or interpretation of data; in writing of the manuscript or in the decision to publish the results.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

