Preparation and Characterization of Cellulose Acetate Propionate Films Functionalized with Reactive Ionic Liquids
- PMID: 31330836
- PMCID: PMC6680812
- DOI: 10.3390/polym11071217
Preparation and Characterization of Cellulose Acetate Propionate Films Functionalized with Reactive Ionic Liquids
Abstract
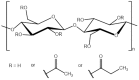
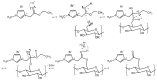
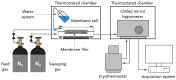
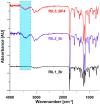
1-(1,3-diethoxy-1,3-dioxopropan-2-ylo)-3-methylimidazolium bromide (RIL1_Br), 1-(2-etoxy-2-oxoethyl)-3-methylimidazolium bromide (RIL2_Br), 1-(2-etoxy-2-oxoethyl)-3-methylimidazolium tetrafluoroborate (RIL3_BF4) ionic liquids were synthesized. Subsequently, the dense cellulose acetate propionate (CAP)-based materials containing from 9 to 28.6 wt % of these reactive ionic liquids were elaborated. Reactive ionic liquids (RILs) were immobilized in CAP as a result of the transesterification reaction. The yield of this reaction was over 90% with respect to the used RIL. The physicochemical properties of resultant films were studied using nuclear magnetic resonance (NMR), scanning electron microscopy (SEM), energy dispersive X-ray (EDX), atomic force microscopy (AFM), and thermogravimetric analysis (TGA). The RIL incorporation influenced the morphology of films by increasing their surface roughness with the rise of RIL content. The thermal stability of CAP-based membranes was dependent on the nature of the ionic liquid. Nevertheless, it was proven that CAP films containing RILs were stable up to 120-150 °C. Transport properties were characterized by water permeation tests. It was found that the type and the amount of the ionic liquid in the CAP matrix substantially influenced the transport properties of the prepared hybrid materials.
Keywords: cellulose acetate propionate; material characterization; polymer membranes; reactive ionic liquid; transesterification reaction; water transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Bolto B., Tran T., Hoang M., Xie Z. Crosslinked poly(vinyl alcohol) membranes. Prog. Polym. Sci. 2009;34:969–981. doi: 10.1016/j.progpolymsci.2009.05.003. - DOI
-
- Ye Y.-S., Rick J., Hwang B.-J. Ionic liquid polymer electrolytes. J. Mater. Chem. A. 2013;1:2719–2743. doi: 10.1039/C2TA00126H. - DOI
-
- Baker R.W. Membrane Technology and Applications. 2nd ed. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2004.
-
- Ulbricht M. Advanced functional polymer membranes. Polymer. 2006;47:2217–2262. doi: 10.1016/j.polymer.2006.01.084. - DOI
-
- Khulbe K.C., Feng C., Matsuura T. The art of surface modification of synthetic polymeric membranes. J. Appl. Polym. Sci. 2010;115:855–895. doi: 10.1002/app.31108. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

