Faster Gastric Emptying Is Unrelated to Feeding Success in Preterm Infants: Randomized Controlled Trial
- PMID: 31330882
- PMCID: PMC6683060
- DOI: 10.3390/nu11071670
Faster Gastric Emptying Is Unrelated to Feeding Success in Preterm Infants: Randomized Controlled Trial
Abstract
Objectives: To evaluate the relationship between gastric emptying (GE) time and days to achievement of full enteral feeding (≥140 mL/kg/day) in preterm infants randomly assigned to receive one of two marketed study formulas for the first 14 feeding days: intact protein premature formula (IPF) or extensively hydrolyzed protein (EHF) formula.
Methods: In this triple-blind, controlled, prospective, clinical trial, we report GE time (time to half-emptying, t1/2) by real-time ultrasonography on Study Day 14, in preterm infants receiving IPF or EHF formula. The association between GE time and achievement of full enteral feeding was evaluated by Pearson correlation. Per-protocol populations for analysis included participants who (1) completed the study (overall) and (2) who received ≥ 75% study formula intake (mL/kg/day).
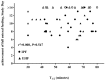
Results: Median GE time at Day 14 was significantly faster for the EHF vs. IPF group overall and in participants who received ≥ 75% study formula intake (p ≤ 0.018). However, we demonstrated GE time had no correlation with the achievement of full enteral feeding (r = 0.08; p = 0.547).
Conclusion: Feeding IP premature formula vs. EH formula was associated with shorter time to full enteral feeding. However, faster GE time did not predict feeding success and may not be a clinically relevant surrogate for assessing feeding tolerance.
Keywords: feeding tolerance; gastric emptying; infant; infant formula; premature; ultrasonography.
Conflict of interest statement
Baldassarre, Montagna, Di Mauro, Laforgia, Capozza and Fanelli’s institution was provided funding in order to independently enroll infants and coordinate the study. Baldassarre was provided travel funds to present some of the data. Cooper is an employee of Mead Johnson Nutrition in the Department of Medical Affairs.
Figures


References
-
- Lapillonne A., Matar M., Adleff A., Chbihi M., Kermorvant-Duchemin E., Campeotto F. Use of extensively hydrolysed formula for refeeding neonates postnecrotising enterocolitis: A nationwide survey-based, cross-sectional study. BMJ Open. 2016;6:e008613. doi: 10.1136/bmjopen-2015-008613. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

