Intramolecular Carbene C-H Insertion Reactions of 2-Diazo-2-sulfamoylacetamides
- PMID: 31330952
- PMCID: PMC6680402
- DOI: 10.3390/molecules24142628
Intramolecular Carbene C-H Insertion Reactions of 2-Diazo-2-sulfamoylacetamides
Abstract
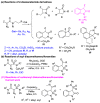
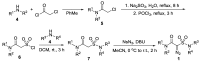
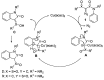
The intramolecular C-H insertions of carbenes derived from 2-diazo-2-sulfamoylacetamides were studied. 2-Diazo-2-sulfamoylacetamides were first prepared from chloroacetyl chloride and secondary amines through acylation followed by sequential treatments with sodium sulfite, phosphorus oxychloride, secondary amines, and 4-nitrobenzenesulfonyl azide. The results indicate that: (1) 2-diazo-N,N-dimethyl-2-(N,N-diphenylsulfamoyl)acetamide can take the formal aromatic 1,5-C-H insertion in its N-phenylsulfonamide moiety to afford the corresponding 1,3-dihydrobenzo[c]isothiazole-3-carboxamide 2,2-dioxide derivative; (2) no aliphatic C-H insertions occur for 2-diazo-2-(N,N-dialkylsulfamoyl)acetamides; and (3) for 2-diazo-N-phenyl-2-(N-phenylsulfamoyl)acetamides, the formal aromatic 1,5-C-H insertion in the N-phenylacetamide moiety is favorable to afford the corresponding 3-sulfamoylindolin-2-one derivatives as sole or major products. The intramolecular competitive aromatic 1,5-C-H insertion reactions of 2-diazo-2-sulfamoylacetamides with aryl groups on both amide and sulfonamide groups reveal that the N-aryl substituents on acetamide are more active than those on sulfonamide. The chemoselectivity is controlled by electronic effect of the aryl group.
Keywords: C-H insertion; amide; carbene insertion; competitive reaction; diazo compound; sulfonamide; sultam.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Padwa A., Krunpe K.E. Application of Intramolecular Carbenoid Reactions in Organic Synthesis. Tetrahedron. 1992;48:5385–5453. doi: 10.1016/S0040-4020(01)88298-3. - DOI
-
- Doyle M.P., McKervey M.A., Ye T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds. John Willey and Sons; New York, NY, USA: 2008.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources