Assessing the Lifetime Cost-Effectiveness of Low-Protein Infant Formula as Early Obesity Prevention Strategy: The CHOP Randomized Trial
- PMID: 31331027
- PMCID: PMC6682975
- DOI: 10.3390/nu11071653
Assessing the Lifetime Cost-Effectiveness of Low-Protein Infant Formula as Early Obesity Prevention Strategy: The CHOP Randomized Trial
Abstract
Background: Although there is a growing number of early childhood obesity prevention programs, only a few of them are effective in the long run. Even fewer reports exist on lifetime cost-effectiveness of early prevention strategies. This paper aimed to assess the lifetime cost-effectiveness of infant feeding modification aiming at reducing risk of later obesity.
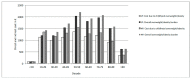
Methods: The simulation model consists of two parts: (a) Model I used data from the European Childhood Obesity Project (CHOP) trial (up to 6 years) and the German Interview and Examination Survey for Children (KiGGS) (6-17 years) to evaluate BMI trajectories of infants receiving either lower protein (LP) or higher protein (HP) content formula; and (b) Model II estimated lifetime cost-effectiveness based on Model I BMI trajectories. Compared to HP formula, LP formula feeding would incur lower costs that are attributable to childhood obesity across all decades of life.
Results: Our analysis showed that LP formula would be cost-effective in terms of a positive net monetary benefit (discounted 3%) as an obesity prevention strategy. For the 19% of infants fed with formula in Germany, the LP strategy would result in cost savings of € 2.5 billion.
Conclusions: Our study is one of the first efforts to provide much-needed cost-effectiveness evidence of infant feeding modification, thereby potentially motivating interventionists to reassess their resource allocation.
Keywords: childhood; cost-effectiveness; early nutrition; formula; markov model; obesity prevention.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Fernandes M.M. Ph.D. Thesis. Pardee RAND Graduate School; Santa Monica, CA, USA: 2010. Estimating the Lifecycle Costs Associated with Childhood Obesity in Evaluating the Impacts of School Nutrition and Physical Activity Policies on Child Health.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

