Macrophage-Mediated Subversion of Anti-Tumour Immunity
- PMID: 31331034
- PMCID: PMC6678757
- DOI: 10.3390/cells8070747
Macrophage-Mediated Subversion of Anti-Tumour Immunity
Abstract
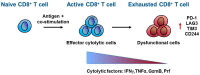
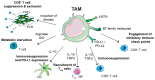
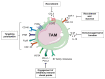
Despite the incredible clinical benefits obtained by the use of immune checkpoint blockers (ICBs), resistance is still common for many types of cancer. Central for ICBs to work is activation and infiltration of cytotoxic CD8+ T cells following tumour-antigen recognition. However, it is now accepted that even in the case of immunogenic tumours, the effector functions of CD8+ T cells are highly compromised by the presence of an immunosuppressive tumour microenvironment (TME) at the tumour site. Tumour-associated macrophages (TAMs) are among the most abundant non-malignant stromal cell types within the TME and they are crucial drivers of tumour progression, metastasis and resistance to therapy. TAMs are able to regulate either directly or indirectly various aspects of tumour immunity, including T cell recruitment and functions. In this review we discuss the mechanisms by which TAMs subvert CD8+ T cell immune surveillance and how their targeting in combination with ICBs represents a very powerful therapeutic strategy.
Keywords: anti-tumour immunity; cancer; immune checkpoint blockers; immunosuppression; macrophage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

