HLA-DQA1 and HLA-DQB1 Alleles, Conferring Susceptibility to Celiac Disease and Type 1 Diabetes, are More Expressed Than Non-Predisposing Alleles and are Coordinately Regulated
- PMID: 31331105
- PMCID: PMC6678473
- DOI: 10.3390/cells8070751
HLA-DQA1 and HLA-DQB1 Alleles, Conferring Susceptibility to Celiac Disease and Type 1 Diabetes, are More Expressed Than Non-Predisposing Alleles and are Coordinately Regulated
Abstract
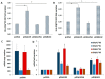
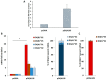
HLA DQA1*05 and DQB1*02 alleles encoding the DQ2.5 molecule and HLA DQA1*03 and DQB1*03 alleles encoding DQ8 molecules are strongly associated with celiac disease (CD) and type 1 diabetes (T1D), two common autoimmune diseases (AD). We previously demonstrated that DQ2.5 genes showed a higher expression with respect to non-CD associated alleles in heterozygous DQ2.5 positive (HLA DR1/DR3) antigen presenting cells (APC) of CD patients. This differential expression affected the level of the encoded DQ2.5 molecules on the APC surface and established the strength of gluten-specific CD4+ T cells response. Here, we expanded the expression analysis of risk alleles in patients affected by T1D or by T1D and CD comorbidity. In agreement with previous findings, we found that DQ2.5 and DQ8 risk alleles are more expressed than non-associated alleles also in T1D patients and favor the self-antigen presentation. To investigate the mechanism causing the high expression of risk alleles, we focused on HLA DQA1*05 and DQB1*02 alleles and, by ectopic expression of a single mRNA, we modified the quantitative equilibrium among the two transcripts. After transfection of DR7/DR14 B-LCL with HLA-DQA1*05 cDNA, we observed an overexpression of the endogenous DQB1*02 allele. The DQ2.5 heterodimer synthesized was functional and able to present gluten antigens to cognate CD4+ T cells. Our results indicated that the high expression of alpha and beta transcripts, encoding for the DQ2.5 heterodimeric molecules, was strictly coordinated by a mechanism acting at a transcriptional level. These findings suggested that, in addition to the predisposing HLA-DQ genotype, also the expression of risk alleles contributed to the establishment of autoimmunity.
Keywords: autoimmunity; expression; regulation; risk genes.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



Similar articles
-
Risk genes and autoantibodies in Egyptian children with type 1 diabetes - low frequency of autoantibodies in carriers of the HLA-DRB1*04:05-DQA1*03-DQB1*02 risk haplotype.Diabetes Metab Res Rev. 2015 Mar;31(3):287-94. doi: 10.1002/dmrr.2609. Epub 2014 Nov 24. Diabetes Metab Res Rev. 2015. PMID: 25256132
-
Differential expression of predisposing HLA-DQ2.5 alleles in DR5/DR7 celiac disease patients affects the pathological immune response to gluten.Sci Rep. 2020 Oct 14;10(1):17227. doi: 10.1038/s41598-020-73907-2. Sci Rep. 2020. PMID: 33057065 Free PMC article.
-
HLA-DQ genetic risk gradient for type 1 diabetes and celiac disease in northwestern Mexico.Rev Gastroenterol Mex. 2015 Apr-Jun;80(2):135-43. doi: 10.1016/j.rgmx.2015.03.003. Epub 2015 Jun 16. Rev Gastroenterol Mex. 2015. PMID: 26088570 English, Spanish.
-
HLA-DQA1 and HLA-DQB1 in Celiac disease predisposition: practical implications of the HLA molecular typing.J Biomed Sci. 2012 Oct 11;19(1):88. doi: 10.1186/1423-0127-19-88. J Biomed Sci. 2012. PMID: 23050549 Free PMC article. Review.
-
Genetic susceptibilty and celiac disease: what role do HLA haplotypes play?Acta Biomed. 2018 Dec 17;89(9-S):17-21. doi: 10.23750/abm.v89i9-S.7953. Acta Biomed. 2018. PMID: 30561391 Free PMC article. Review.
Cited by
-
The frequency of HLA-DQ2/DQ8 haplotypes and celiac disease among the first-degree relatives of patients with celiac disease.Gastroenterol Hepatol Bed Bench. 2021 Winter;14(1):36-43. Gastroenterol Hepatol Bed Bench. 2021. PMID: 33868608 Free PMC article.
-
Decoding Diabetes Biomarkers and Related Molecular Mechanisms by Using Machine Learning, Text Mining, and Gene Expression Analysis.Int J Environ Res Public Health. 2022 Oct 26;19(21):13890. doi: 10.3390/ijerph192113890. Int J Environ Res Public Health. 2022. PMID: 36360783 Free PMC article.
-
Admixture as a source for HLA variation in Neolithic European farming communities.Genome Biol. 2025 Feb 28;26(1):43. doi: 10.1186/s13059-025-03509-6. Genome Biol. 2025. PMID: 40022192 Free PMC article.
-
Association Between Type 1 Diabetes Mellitus and Celiac Disease: Autoimmune Disorders With a Shared Genetic Background.Cureus. 2022 Mar 7;14(3):e22912. doi: 10.7759/cureus.22912. eCollection 2022 Mar. Cureus. 2022. PMID: 35399440 Free PMC article. Review.
-
New Evidence in the Pathogenesis of Celiac Disease and Type 1 Diabetes Mellitus: A Systematic Review.Cureus. 2021 Jul 29;13(7):e16721. doi: 10.7759/cureus.16721. eCollection 2021 Jul. Cureus. 2021. PMID: 34513356 Free PMC article. Review.
References
-
- Van Lummel M., Duinkerken G., Van Veelen P.A., De Ru A., Cordfunke R., Zaldumbide A., Gomez-Touriño I., Arif S., Peakman M., Drijfhout J.W., et al. Posttranslational modification of HLA-DQ binding islet autoantigens in type 1 diabetes. Diabetes. 2014;63:237–247. doi: 10.2337/db12-1214. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

