Antibiotics inhibit tumor and disease activity in cutaneous T-cell lymphoma
- PMID: 31331920
- PMCID: PMC6764271
- DOI: 10.1182/blood.2018888107
Antibiotics inhibit tumor and disease activity in cutaneous T-cell lymphoma
Abstract
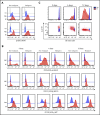
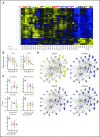
It has been proposed that CD4 T-cell responses to Staphylococcus aureus (SA) can inadvertently enhance neoplastic progression in models of skin cancer and cutaneous T-cell lymphoma (CTCL). In this prospective study, we explored the effect of transient antibiotic treatment on tumor cells and disease activity in 8 patients with advanced-stage CTCL. All patients experienced significant decrease in clinical symptoms in response to aggressive, transient antibiotic treatment. In some patients, clinical improvements lasted for more than 8 months. In 6 of 8 patients, a malignant T-cell clone could be identified in lesional skin, and a significant decrease in the fraction of malignant T cells was observed following antibiotics but an otherwise unchanged treatment regimen. Immunohistochemistry, global messenger RNA expression, and cell-signaling pathway analysis indicated that transient aggressive antibiotic therapy was associated with decreased expression of interleukin-2 high-affinity receptors (CD25), STAT3 signaling, and cell proliferation in lesional skin. In conclusion, this study provides novel evidence suggesting that aggressive antibiotic treatment inhibits malignant T cells in lesional skin. Thus, we provide a novel rationale for treatment of SA in advanced CTCL.
Conflict of interest statement
Conflict-of-interest disclosure: T.L. is employed both by Copenhagen University and by LEO Pharma A/S. J.C.B. has received advisory board honoraria from Takeda. The remaining authors declare no competing financial interests.
Figures



Comment in
-
Antibiotics can improve CTCL.Blood. 2019 Sep 26;134(13):1000-1001. doi: 10.1182/blood.2019002236. Blood. 2019. PMID: 31558556 No abstract available.
-
The Novel Role of Antibiotic Treatment in the Management of Cutaneous T-Cell Lymphoma (CTCL) Patients.J Cutan Med Surg. 2020 Jul/Aug;24(4):410-411. doi: 10.1177/1203475420926981. Epub 2020 May 12. J Cutan Med Surg. 2020. PMID: 32394726 No abstract available.
References
-
- Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350(19):1978-1988. - PubMed
-
- Sommer VH, Clemmensen OJ, Nielsen O, et al. In vivo activation of STAT3 in cutaneous T-cell lymphoma. Evidence for an antiapoptotic function of STAT3. Leukemia. 2004;18(7):1288-1295. - PubMed
-
- Pérez C, Mondéjar R, García-Díaz N, et al. Advanced-stage Mycosis fungoides. Role of STAT3, NFKB and NFAT pathways. [published online ahead of print 3 May 2019]. Br J Dermatol.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

