Dissection of GTPase-activating proteins reveals functional asymmetry in the COPI coat of budding yeast
- PMID: 31331965
- PMCID: PMC6737914
- DOI: 10.1242/jcs.232124
Dissection of GTPase-activating proteins reveals functional asymmetry in the COPI coat of budding yeast
Abstract
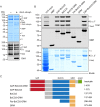
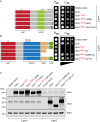
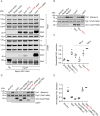
The Arf GTPase controls formation of the COPI vesicle coat. Recent structural models of COPI revealed the positioning of two Arf1 molecules in contrasting molecular environments. Each of these pockets for Arf1 is expected to also accommodate an Arf GTPase-activating protein (ArfGAP). Structural evidence and protein interactions observed between isolated domains indirectly suggest that each niche preferentially recruits one of the two ArfGAPs known to affect COPI, i.e. Gcs1/ArfGAP1 and Glo3/ArfGAP2/3, although only partial structures are available. The functional role of the unique non-catalytic domain of either ArfGAP has not been integrated into the current COPI structural model. Here, we delineate key differences in the consequences of triggering GTP hydrolysis through the activity of one versus the other ArfGAP. We demonstrate that Glo3/ArfGAP2/3 specifically triggers Arf1 GTP hydrolysis impinging on the stability of the COPI coat. We show that the Snf1 kinase complex, the yeast homologue of AMP-activated protein kinase (AMPK), phosphorylates the region of Glo3 that is crucial for this effect and, thereby, regulates its function in the COPI-vesicle cycle. Our results revise the model of ArfGAP function in the molecular context of COPI.This article has an associated First Person interview with the first author of the paper.
Keywords: AMP kinase; Arf GTPase; ArfGAP; COPI vesicle; Gcs1; Glo3.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

