Trans-endocytosis elicited by nectins transfers cytoplasmic cargo, including infectious material, between cells
- PMID: 31331966
- PMCID: PMC6737912
- DOI: 10.1242/jcs.235507
Trans-endocytosis elicited by nectins transfers cytoplasmic cargo, including infectious material, between cells
Abstract
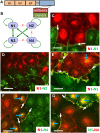
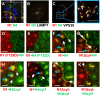
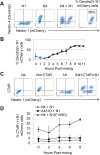
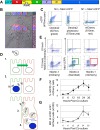
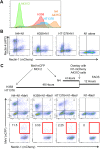
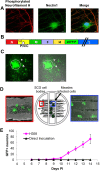
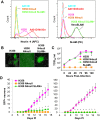
Here, we show that cells expressing the adherens junction protein nectin-1 capture nectin-4-containing membranes from the surface of adjacent cells in a trans-endocytosis process. We find that internalized nectin-1-nectin-4 complexes follow the endocytic pathway. The nectin-1 cytoplasmic tail controls transfer: its deletion prevents trans-endocytosis, while its exchange with the nectin-4 tail reverses transfer direction. Nectin-1-expressing cells acquire dye-labeled cytoplasmic proteins synchronously with nectin-4, a process most active during cell adhesion. Some cytoplasmic cargo remains functional after transfer, as demonstrated with encapsidated genomes of measles virus (MeV). This virus uses nectin-4, but not nectin-1, as a receptor. Epithelial cells expressing nectin-4, but not those expressing another MeV receptor in its place, can transfer infection to nectin-1-expressing primary neurons. Thus, this newly discovered process can move cytoplasmic cargo, including infectious material, from epithelial cells to neurons. We name the process nectin-elicited cytoplasm transfer (NECT). NECT-related trans-endocytosis processes may be exploited by pathogens to extend tropism. This article has an associated First Person interview with the first author of the paper.
Keywords: Cell adhesion; Cell communication; Cytoplasm transfer; Measles virsus; Nectin; Neuronal entry; Trans-endocytosis; Virus receptor.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
References
-
- Bellini W. J., Rota J. S., Lowe L. E., Katz R. S., Dyken P. R., Zaki S. R., Shieh W. J. and Rota P. A. (2005). Subacute sclerosing panencephalitis: more cases of this fatal disease are prevented by measles immunization than was previously recognized. J. Infect. Dis. 192, 1686-1693. 10.1086/497169 - DOI - PubMed
-
- Bonaparte M. I., Dimitrov A. S., Bossart K. N., Crameri G., Mungall B. A., Bishop K. A., Choudhry V., Dimitrov D. S., Wang L.-F., Eaton B. T. et al. (2005). Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus. Proc. Natl. Acad. Sci. USA 102, 10652-10657. 10.1073/pnas.0504887102 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

