Glutathione Synthesis Contributes to Virulence of Streptococcus agalactiae in a Murine Model of Sepsis
- PMID: 31331978
- PMCID: PMC6755738
- DOI: 10.1128/JB.00367-19
Glutathione Synthesis Contributes to Virulence of Streptococcus agalactiae in a Murine Model of Sepsis
Abstract
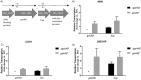
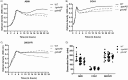
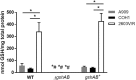
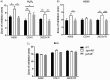
Streptococcus agalactiae, a leading cause of sepsis and meningitis in neonates, utilizes multiple virulence factors to survive and thrive within the human host during an infection. Unique among the pathogenic streptococci, S. agalactiae uses a bifunctional enzyme encoded by a single gene (gshAB) to synthesize glutathione (GSH), a major antioxidant in most aerobic organisms. Since S. agalactiae can also import GSH, similar to all other pathogenic streptococcal species, the contribution of GSH synthesis to the pathogenesis of S. agalactiae disease is not known. In the present study, gshAB deletion mutants were generated in strains representing three of the most prevalent clinical serotypes of S. agalactiae and were compared against isogenic wild-type and gshAB knock-in strains. When cultured in vitro in a chemically defined medium under nonstress conditions, each mutant and its corresponding wild type had comparable growth rates, generation times, and growth yields. However, gshAB deletion mutants were found to be more sensitive than wild-type or gshAB knock-in strains to killing and growth inhibition by several different reactive oxygen species. Furthermore, deletion of gshAB in S. agalactiae strain COH1 significantly attenuated virulence compared to the wild-type or gshAB knock-in strains in a mouse model of sepsis. Taken together, these data establish that GSH is a virulence factor important for resistance to oxidative stress and that de novo GSH synthesis plays a crucial role in S. agalactiae pathogenesis and further suggest that the inhibition of GSH synthesis may provide an opportunity for the development of novel therapies targeting S. agalactiae disease.IMPORTANCE Approximately 10 to 30% of women are naturally and asymptomatically colonized by Streptococcus agalactiae However, transmission of S. agalactiae from mother to newborn during vaginal birth is a leading cause of neonatal meningitis. Although colonized mothers who are at risk for transmission to the newborn are treated with antibiotics prior to delivery, S. agalactiae is becoming increasingly resistant to current antibiotic therapies, and new treatments are needed. This research reveals a critical stress resistance pathway, glutathione synthesis, that is utilized by S. agalactiae and contributes to its pathogenesis. Understanding the role of this unique bifunctional glutathione synthesis enzyme in S. agalactiae during sepsis may help elucidate why S. agalactiae produces such an abundance of glutathione compared to other bacteria.
Keywords: Streptococcus agalactiae; glutathione; hydrogen peroxide; hypochlorous acid; oxidative stress; virulence.
Copyright © 2019 American Society for Microbiology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

