Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: one year results from the first international, open-label, prospective, multicentre study
- PMID: 31331994
- PMCID: PMC6984054
- DOI: 10.1136/gutjnl-2019-318349
Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: one year results from the first international, open-label, prospective, multicentre study
Abstract
Background: The duodenum has become a metabolic treatment target through bariatric surgery learnings and the specific observation that bypassing, excluding or altering duodenal nutrient exposure elicits favourable metabolic changes. Duodenal mucosal resurfacing (DMR) is a novel endoscopic procedure that has been shown to improve glycaemic control in people with type 2 diabetes mellitus (T2D) irrespective of body mass index (BMI) changes. DMR involves catheter-based circumferential mucosal lifting followed by hydrothermal ablation of duodenal mucosa. This multicentre study evaluates safety and feasibility of DMR and its effect on glycaemia at 24 weeks and 12 months.
Methods: International multicentre, open-label study. Patients (BMI 24-40) with T2D (HbA1c 59-86 mmol/mol (7.5%-10.0%)) on stable oral glucose-lowering medication underwent DMR. Glucose-lowering medication was kept stable for at least 24 weeks post DMR. During follow-up, HbA1c, fasting plasma glucose (FPG), weight, hepatic transaminases, Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), adverse events (AEs) and treatment satisfaction were determined and analysed using repeated measures analysis of variance with Bonferroni correction.
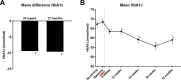
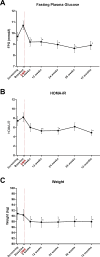
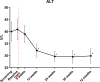
Results: Forty-six patients were included of whom 37 (80%) underwent complete DMR and 36 were finally analysed; in remaining patients, mainly technical issues were observed. Twenty-four patients had at least one AE (52%) related to DMR. Of these, 81% were mild. One SAE and no unanticipated AEs were reported. Twenty-four weeks post DMR (n=36), HbA1c (-10±2 mmol/mol (-0.9%±0.2%), p<0.001), FPG (-1.7±0.5 mmol/L, p<0.001) and HOMA-IR improved (-2.9±1.1, p<0.001), weight was modestly reduced (-2.5±0.6 kg, p<0.001) and hepatic transaminase levels decreased. Effects were sustained at 12 months. Change in HbA1c did not correlate with modest weight loss. Diabetes treatment satisfaction scores improved significantly.
Conclusions: In this multicentre study, DMR was found to be a feasible and safe endoscopic procedure that elicited durable glycaemic improvement in suboptimally controlled T2D patients using oral glucose-lowering medication irrespective of weight loss. Effects on the liver are examined further.
Trial registration number: NCT02413567.
Keywords: diabetes mellitus; duodenal mucosa; endoscopic procedures; glucose metabolism; therapeutic endoscopy.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: FH reports speaker fees from Sanofi, Bioton and Astra Zeneca. DH reports consultancy for Novo Nordisk, Sanofi and Roche and speaker fees from Novo Nordisk, Sanofi, Roche, Astra Zeneca, Boerhinger, Napp, Medtronic, Sunovion and Fractyl Laboratories. LRG reports consultancy for Fractyl Laboratories. MGN reports consultancy for Fractyl Laboratories, GI Dynamics, GI Windows, Ethicon EndoSurgery, Meditronics, Apollo EndoSurgery, Consultant and Scientific Advisory Board member for GI Dynamics and Faculty in training courses for Ethicon EndoSurgery and Meditronics.
Figures

Comment in
-
Reduction of HbA1c in patients with type 2 diabetes following duodenal mucosal resurfacing: could other factors be at play?Gut. 2020 Dec;69(12):2260-2261. doi: 10.1136/gutjnl-2020-320900. Epub 2020 Feb 26. Gut. 2020. PMID: 32102927 No abstract available.
-
Response to letter titled 'Reduction of HbA1c in patients with type 2 diabetes following duodenal mucosal resurfacing: could other factors be at play?'.Gut. 2021 Jan;70(1):218. doi: 10.1136/gutjnl-2020-321170. Epub 2020 Mar 30. Gut. 2021. PMID: 32229545 Free PMC article. No abstract available.
References
-
- International Diabetes Federation. IDF Diabetes Atlas. 6th edn, 2014.
-
- Inzucchi SE, Bergenstal RM, Buse JB, et al. . Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015;58:429–42. 10.1007/s00125-014-3460-0 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
