Human species-specific loss of CMP- N-acetylneuraminic acid hydroxylase enhances atherosclerosis via intrinsic and extrinsic mechanisms
- PMID: 31332008
- PMCID: PMC6690033
- DOI: 10.1073/pnas.1902902116
Human species-specific loss of CMP- N-acetylneuraminic acid hydroxylase enhances atherosclerosis via intrinsic and extrinsic mechanisms
Abstract
Cardiovascular disease (CVD) events due to atherosclerosis cause one-third of worldwide deaths and risk factors include physical inactivity, age, dyslipidemia, hypertension, diabetes, obesity, smoking, and red meat consumption. However, ∼15% of first-time events occur without such factors. In contrast, coronary events are extremely rare even in closely related chimpanzees in captivity, despite human-like CVD-risk-prone blood lipid profiles, hypertension, and mild atherosclerosis. Similarly, red meat-associated enhancement of CVD event risk does not seem to occur in other carnivorous mammals. Thus, heightened CVD risk may be intrinsic to humans, and genetic changes during our evolution need consideration. Humans exhibit a species-specific deficiency of the sialic acid N-glycolylneuraminic acid (Neu5Gc), due to pseudogenization of cytidine monophosphate-N-acetylneuraminic acid (Neu5Ac) hydroxylase (CMAH), which occurred in hominin ancestors ∼2 to 3 Mya. Ldlr-/- mice with human-like Cmah deficiency fed a sialic acids (Sias)-free high-fat diet (HFD) showed ∼1.9-fold increased atherogenesis over Cmah wild-type Ldlr-/- mice, associated with elevated macrophage cytokine expression and enhanced hyperglycemia. Human consumption of Neu5Gc (from red meat) acts as a "xeno-autoantigen" via metabolic incorporation into endogenous glycoconjugates, as interactions with circulating anti-Neu5Gc "xeno-autoantibodies" potentiate chronic inflammation ("xenosialitis"). Cmah-/-Ldlr-/- mice immunized with Neu5Gc-bearing antigens to generate human-like anti-Neu5Gc antibodies suffered a ∼2.4-fold increased atherosclerosis on a Neu5Gc-rich HFD, compared with Neu5Ac-rich or Sias-free HFD. Lesions in Neu5Gc-immunized and Neu5Gc-rich HFD-fed Cmah-/-Ldlr-/- mice were more advanced but unexplained by lipoprotein or glucose changes. Human evolutionary loss of CMAH likely contributes to atherosclerosis predisposition via multiple intrinsic and extrinsic mechanisms, and future studies could consider this more human-like model.
Keywords: CMAH; N-glycolylneuraminic acid (Neu5Gc); atherosclerosis; cytidine-5′-monophosphate (CMP)-N-acetylneuraminic acid (Neu5Ac) hydroxylase (CMAH); human evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

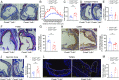
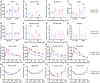
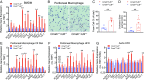


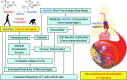
Comment in
-
Enzyme loss during evolution linked to atherosclerosis predisposition.Nat Rev Cardiol. 2019 Oct;16(10):580. doi: 10.1038/s41569-019-0251-9. Nat Rev Cardiol. 2019. PMID: 31388139 No abstract available.
-
Reply to Soulillou et al.: Difficulties in extrapolating from animal models exemplify unusual human atherosclerosis susceptibility and mechanisms via CMAH loss.Proc Natl Acad Sci U S A. 2020 Jan 28;117(4):1847-1848. doi: 10.1073/pnas.1917278117. Epub 2020 Jan 21. Proc Natl Acad Sci U S A. 2020. PMID: 31964837 Free PMC article. No abstract available.
-
Can we extrapolate from a Cmah -/- Ldlr -/- mouse model a susceptibility for atherosclerosis in humans?Proc Natl Acad Sci U S A. 2020 Jan 28;117(4):1845-1846. doi: 10.1073/pnas.1915658117. Epub 2020 Jan 21. Proc Natl Acad Sci U S A. 2020. PMID: 31964838 Free PMC article. No abstract available.
References
-
- Robinson T., et al. , New Zealand Diabetes Cohort Study cardiovascular risk score for people with Type 2 diabetes: Validation in the PREDICT cohort. J. Prim. Health Care 4, 181–188 (2012). - PubMed
-
- Denton D., et al. , The effect of increased salt intake on blood pressure of chimpanzees. Nat. Med. 1, 1009–1016 (1995). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

