Plant defenses interact with insect enteric bacteria by initiating a leaky gut syndrome
- PMID: 31332013
- PMCID: PMC6689943
- DOI: 10.1073/pnas.1908748116
Plant defenses interact with insect enteric bacteria by initiating a leaky gut syndrome
Abstract
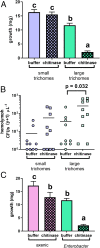
Plants produce suites of defenses that can collectively deter and reduce herbivory. Many defenses target the insect digestive system, with some altering the protective peritrophic matrix (PM) and causing increased permeability. The PM is responsible for multiple digestive functions, including reducing infections from potential pathogenic microbes. In our study, we developed axenic and gnotobiotic methods for fall armyworm (Spodoptera frugiperda) and tested how particular members present in the gut community influence interactions with plant defenses that can alter PM permeability. We observed interactions between gut bacteria with plant resistance. Axenic insects grew more but displayed lower immune-based responses compared with those possessing Enterococcus, Klebsiella, and Enterobacter isolates from field-collected larvae. While gut bacteria reduced performance of larvae fed on plants, none of the isolates produced mortality when injected directly into the hemocoel. Our results strongly suggest that plant physical and chemical defenses not only act directly upon the insect, but also have some interplay with the herbivore's microbiome. Combined direct and indirect, microbe-mediated assaults by maize defenses on the fall armyworm on the insect digestive and immune system reduced growth and elevated mortality in these insects. These results imply that plant-insect interactions should be considered in the context of potential mediation by the insect gut microbiome.
Keywords: Lepidoptera; chitinase; maize; microbiome; trichome.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Agrawal A. A., Fishbein M., Plant defense syndromes. Ecology 87 (suppl. 7), S132–S149 (2006). - PubMed
-
- Rasmann S., Agrawal A. A., Plant defense against herbivory: Progress in identifying synergism, redundancy, and antagonism between resistance traits. Curr. Opin. Plant Biol. 12, 473–478 (2009). - PubMed
-
- Hay M. E., Kappel Q. E., Fenical W., Synergisms in plant defenses against herbivores—Interactions of chemistry, calcification, and plant-quality. Ecology 75, 1714–1726 (1994).
-
- Mithöfer A., Boland W., Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 63, 431–450 (2012). - PubMed
-
- Schuman M. C., Baldwin I. T., The layers of plant responses to insect herbivores. Annu. Rev. Entomol. 61, 373–394 (2016). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

