Single-Center Investigation of the Pharmacokinetics of WCK 4282 (Cefepime-Tazobactam Combination) in Renal Impairment
- PMID: 31332068
- PMCID: PMC6761517
- DOI: 10.1128/AAC.00873-19
Single-Center Investigation of the Pharmacokinetics of WCK 4282 (Cefepime-Tazobactam Combination) in Renal Impairment
Abstract
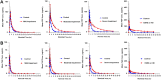
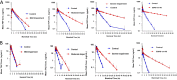
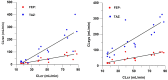
WCK 4282 is a combination product of cefepime (FEP) and tazobactam (TAZ) in a 1:1 ratio currently under development for the treatment of multidrug-resistant Gram-negative bacterial infections. We investigated the effect of renal impairment on the pharmacokinetics (PK) and safety of WCK 4282 in 48 subjects with various degrees of renal function. Subjects were categorized on the basis of their Cockcroft-Gault equation-estimated creatinine clearance (CLCR). We enrolled 6 subjects each into those with mild (CLCR, 60 to <90 ml/min), moderate (CLCR, 30 to <60 ml/min), or severe (CLCR, <30 ml/min) renal impairment and those with end-stage renal disease (ESRD) requiring hemodialysis and 24 healthy control subjects (CLCR, ≥90 ml/min). Healthy subjects and subjects with mild and moderate renal impairment received a single 90-min infusion of 4 g of WCK 4282 (2 g FEP and 2 g TAZ). Subjects with severe renal impairment and ESRD received 2 g of WCK 4282 (1 g FEP and 1 g TAZ) over 90 min. The plasma exposure of FEP-TAZ increased as renal function decreased. In subjects with mild, moderate, and severe renal impairment and ESRD, the mean exposure (area under the plasma concentration versus time curve from time zero extrapolated to infinity) of FEP and TAZ increased by 1.3- and 1.2-fold, 2.3- and 2.3-fold, 4.7- and 4.0-fold, and 8.5- and 11.6-fold, respectively. The urinary recovery of FEP and TAZ decreased with increasing renal impairment. There were no adverse events reported during the study. The findings suggest that dose adjustments for WCK 4282 will be required according to the degree of renal impairment. A single infusion of WCK 4282 was found to be safe and well tolerated in subjects with normal and impaired renal function. (This study has been registered at ClinicalTrials.gov under identifier NCT02709382.).
Keywords: Gram-negative bacterial infections; antibacterial agents; bacterial; multidrug resistance; β-lactamases.
Copyright © 2019 American Society for Microbiology.
Figures

References
-
- Sader HS, Castanheira M, Mendes RE, Flamm RK, Jones RN. 2017. Antimicrobial activity of high-proportion cefepime-tazobactam (WCK 4282) against a large number of Gram-negative isolates collected worldwide in 2014. Antimicrob Agents Chemother 61:e02409-16. doi: 10.1128/AAC.02409-16. - DOI - PMC - PubMed
-
- Castanheira M, Duncan LR, Rhomberg PR, Sader HS. 2017. Enhanced activity of cefepime-tazobactam (WCK 4282) against KPC-producing Enterobacteriaceae when tested in media supplemented with human serum or sodium chloride. Diagn Microbiol Infect Dis 89:305–309. doi: 10.1016/j.diagmicrobio.2017.08.011. - DOI - PubMed
-
- Riedel S, Huband MD, Sader HS, Flamm RK, Jones RN. 2017. Determination of disk diffusion and MIC quality control guidelines for high-dose cefepime-tazobactam (WCK 4282), a novel antibacterial combination consisting of a β-lactamase inhibitor and a fourth-generation cephalosporin. J Clin Microbiol 55:3130–3134. doi: 10.1128/JCM.00788-17. - DOI - PMC - PubMed
-
- Ghafur A, Tayade A, Kannaian P. 2012. Clinical profile of patients treated with cefepime/tazobactam: a new beta-lactam/beta-lactamase inhibitor combination. J Microbiol Infect Dis 2:79–86. doi: 10.5799/ahinjs.02.2012.03.0049. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

