Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response
- PMID: 31332169
- PMCID: PMC6646332
- DOI: 10.1038/s41467-019-11152-6
Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response
Abstract
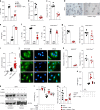
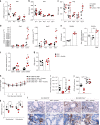
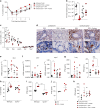
Severe respiratory syncytial virus (RSV) infection is a major cause of morbidity and mortality in infants <2 years-old. Here we describe that high-fiber diet protects mice from RSV infection. This effect was dependent on intestinal microbiota and production of acetate. Oral administration of acetate mediated interferon-β (IFN-β) response by increasing expression of interferon-stimulated genes in the lung. These effects were associated with reduction of viral load and pulmonary inflammation in RSV-infected mice. Type 1 IFN signaling via the IFN-1 receptor (IFNAR) was essential for acetate antiviral activity in pulmonary epithelial cell lines and for the acetate protective effect in RSV-infected mice. Activation of Gpr43 in pulmonary epithelial cells reduced virus-induced cytotoxicity and promoted antiviral effects through IFN-β response. The effect of acetate on RSV infection was abolished in Gpr43-/- mice. Our findings reveal antiviral effects of acetate involving IFN-β in lung epithelial cells and engagement of GPR43 and IFNAR.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

