Performance of the Freestyle Libre flash glucose monitoring (flash GM) system in individuals with type 1 diabetes: A secondary outcome analysis of a randomized crossover trial
- PMID: 31332929
- PMCID: PMC6852439
- DOI: 10.1111/dom.13835
Performance of the Freestyle Libre flash glucose monitoring (flash GM) system in individuals with type 1 diabetes: A secondary outcome analysis of a randomized crossover trial
Abstract
Aims: The efficacy of flash glucose monitoring (flash GM) systems has been demonstrated by improvements in glycaemia; however, during high rates of glucose flux, the performance of continuous glucose monitoring systems was impaired, as detailed in previous studies. This study aimed to determine the performance of the flash GM system during daily-life glycaemic challenges such as carbohydrate-rich meals, bolus insulin-induced glycaemic disturbances and acute physical exercise in individuals with type 1 diabetes.
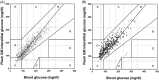
Materials and methods: This study comprised four randomized trial visits with alternating pre- and post-exercise bolus insulin doses. Throughout the four 14-hour inpatient phases, 19 participants received three carbohydrate-rich meals and performed moderate-intensity exercise. Venous blood glucose and capillary blood glucose during exercise was compared to interstitial glucose concentrations. Flash GM accuracy was assessed by median absolute relative difference (MARD) (interquartile range [IQR]) using the Bland-Altman method and Clark error grid, as well as according to guidelines for integrated CGM approvals (Class II-510(K)).
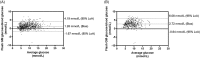
Results: The overall MARD (IQR) during inpatient phases was 14.3% (6.9%-22.8%), during hypoglycaemia (≤3.9 mmol/L) was 31.6% (16.2%-46.8%), during euglycaemia (4.0 mmol/L - 9.9 mmol/L) was 16.0% (8.5%-24.0%) and during hyperglycaemia (≥10 mmol/L) was 9.4% (5.1%-15.7%). Overall Bland-Altman analysis showed a bias (95% LoA) of 1.26 mmol/L (-1.67 to 4.19 mmol/L). The overall MARD during acute exercise was 29.8% (17.5%-39.8%), during hypoglycaemia was 45.1% (35.2%-51.1%), during euglycaemia was 30.7% (18.7%-39.2%) and during hyperglycaemia was 16.3% (10.0%-22.8%).
Conclusion: Flash GM interstitial glucose readings were not sufficiently accurate within the hypoglycaemic range and during acute exercise and require confirmatory blood glucose measurements.
Keywords: continuous glucose monitoring (CGM), exercise intervention, hypoglycaemia, type 1 diabetes.
© 2019 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
O. M. has received lecture fees from Medtronic, travel grants from Novo Nordisk A/S, Novo Nordisk AT, Novo Nordisk UK, Medtronic AT, research grants from Sêr Cymru II COFUND fellowship/European Union, Novo Nordisk A/S and Novo Nordisk AT, as well as material funding from Abbott Diabetes Care. M. L. E. has received a KESS2/European Social Fund scholarship and travel grants from Novo Nordisk A/S. H. S. has received honoraria, travel support or unrestricted research grants from Amgen, Astra Zeneca, Boehringer‐Ingelheim, Eli Lilly, MSD, Novo Nordisk and Sanofi‐Aventis. S. C. B. has received research grants, including those for principal investigator, collaborator or consultant and pending grants, as well as other grants, from Health Care and Research Wales (Welsh Government) and Novo Nordisk; has received research support from Healthcare and Research Wales (Welsh Government), honoraria from Novo Nordisk, Sanofi, Lilly, Boehringer Ingelheim and Merck, and has an ownership interest in Glycosmedia, an on‐line news service concerning diabetes. R. M. B. has received honoraria as well as travel and educational grant support from Boehringer‐Ingelheim, Eli Lilly and Company, Novo Nordisk and Sanofi‐Aventis. The remaining authors have no relevant conflicts of interest to disclose.
Figures
References
-
- Blum JR, Rayfield EJ. An endocrine clinic's perspective and experience with the Abbott Freestyle Libre Cgm. Endocr Pract. 2018;24:309‐311. - PubMed
-
- Bolinder J, Antuna R, Geelhoed‐Duijvestijn P, Kröger J, Weitgasser R. Novel glucose‐sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non‐masked, randomised controlled trial. Lancet. 2016;388:2254‐2263. - PubMed
-
- Edelman SV, Argento NB, Pettus J, Hirsch IB. Clinical implications of real‐time and intermittently scanned continuous glucose monitoring. Diabetes Care. 2018;41:2265‐2274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

