Tissue Response to Neural Implants: The Use of Model Systems Toward New Design Solutions of Implantable Microelectrodes
- PMID: 31333407
- PMCID: PMC6624471
- DOI: 10.3389/fnins.2019.00689
Tissue Response to Neural Implants: The Use of Model Systems Toward New Design Solutions of Implantable Microelectrodes
Abstract
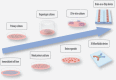
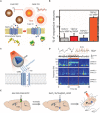
The development of implantable neuroelectrodes is advancing rapidly as these tools are becoming increasingly ubiquitous in clinical practice, especially for the treatment of traumatic and neurodegenerative disorders. Electrodes have been exploited in a wide number of neural interface devices, such as deep brain stimulation, which is one of the most successful therapies with proven efficacy in the treatment of diseases like Parkinson or epilepsy. However, one of the main caveats related to the clinical application of electrodes is the nervous tissue response at the injury site, characterized by a cascade of inflammatory events, which culminate in chronic inflammation, and, in turn, result in the failure of the implant over extended periods of time. To overcome current limitations of the most widespread macroelectrode based systems, new design strategies and the development of innovative materials with superior biocompatibility characteristics are currently being investigated. This review describes the current state of the art of in vitro, ex vivo, and in vivo models available for the study of neural tissue response to implantable microelectrodes. We particularly highlight new models with increased complexity that closely mimic in vivo scenarios and that can serve as promising alternatives to animal studies for investigation of microelectrodes in neural tissues. Additionally, we also express our view on the impact of the progress in the field of neural tissue engineering on neural implant research.
Keywords: brain slice cultures; deep brain stimulation; foreign body reaction; microelectrodes; neural tissue engineering; neural tissue response.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

