Mast Cell/Proteinase Activated Receptor 2 (PAR2) Mediated Interactions in the Pathogenesis of Discogenic Back Pain
- PMID: 31333416
- PMCID: PMC6625229
- DOI: 10.3389/fncel.2019.00294
Mast Cell/Proteinase Activated Receptor 2 (PAR2) Mediated Interactions in the Pathogenesis of Discogenic Back Pain
Abstract
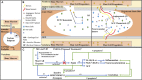
Mast cells (MCs) are present in the painful degenerate human intervertebral disc (IVD) and are associated with disease pathogenesis. MCs release granules containing enzymatic and inflammatory factors in response to stimulants or allergens. The serine protease, tryptase, is unique to MCs and its activation of the G-protein coupled receptor, Protease Activated Receptor 2 (PAR2), induces inflammation and degradation in osteoarthritic cartilage. Our previously published work has demonstrated increased levels of MC marker tryptase in IVD samples from discogenic back pain patients compared to healthy control IVD samples including expression of chemotactic agents that may facilitate MC migration into the IVD. To further elucidate MCs' role in the IVD and mechanisms underlying its effects, we investigated whether (1) human IVD cells can promote MC migration, (2) MC tryptase can mediate up-regulation of inflammatory/catabolic process in human IVD cells and tissue, and (3) the potential of PAR2 antagonist to function as a therapeutic drug in in vitro human and ex vivo bovine pilot models of disease. MC migration was quantitatively assessed using conditioned media from primary human IVD cells and MC migration examined through Matrigel. Exposure to soluble IVD factors significantly enhanced MC migration, suggesting IVD cells can recruit MCs. We also demonstrated significant upregulation of MC chemokine SCF and angiogenic factor VEGFA gene expression in human IVD cells in vitro in response to recombinant human tryptase, suggesting tryptase can enhance recruitment of MCs and promotion of angiogenesis into the usually avascular IVD. Furthermore, tryptase can degrade proteoglycans in IVD tissue as demonstrated by significant increases in glycosaminoglycans released into surrounding media. This can create a catabolic microenvironment compromising structural integrity and facilitating vascular migration usually inhibited by the anti-angiogenic IVD matrix. Finally, as a "proof of concept" study, we examined the therapeutic potential of PAR2 antagonist (PAR2A) on human IVD cells and bovine organ culture IVD model. While preliminary data shows promise and points toward structural restoration of the bovine IVD including down-regulation of VEGFA, effects of PAR2 antagonist on human IVD cells differ between gender and donors suggesting that further validation is required with larger cohorts of human specimens.
Keywords: PAR2; discogenic back pain; intervertebral disc; mast cell; tryptase.
Figures




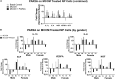
References
-
- Bartynski W. S., Dejohn L. M., Rothfus W. E., Gerszten P. C. (2013). “Progressive-Onset” versus injury-associated discogenic low back pain: features of disc internal derangement in patients studied with provocation lumbar discography. Interv. Neuroradiol. 19 110–120. 10.1177/159101991301900117 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

