Ginkgo Flavonol Glycosides or Ginkgolides Tend to Differentially Protect Myocardial or Cerebral Ischemia-Reperfusion Injury via Regulation of TWEAK-Fn14 Signaling in Heart and Brain
- PMID: 31333457
- PMCID: PMC6624656
- DOI: 10.3389/fphar.2019.00735
Ginkgo Flavonol Glycosides or Ginkgolides Tend to Differentially Protect Myocardial or Cerebral Ischemia-Reperfusion Injury via Regulation of TWEAK-Fn14 Signaling in Heart and Brain
Abstract
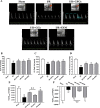
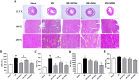
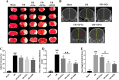
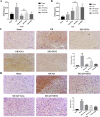
Shuxuening injection (SXNI), one of the pharmaceutical preparations of Ginkgo biloba extract, has significant effects on both ischemic stroke and heart diseases from bench to bedside. Its major active ingredients are ginkgo flavonol glycosides (GFGs) and ginkgolides (GGs). We have previously reported that SXNI as a whole protected ischemic brain and heart, but the active ingredients and their contribution to the therapeutic effects remain unclear. Therefore, we combined experimental and network analysis approach to further explore the specific effects and underlying mechanisms of GFGs and GGs of SXNI on ischemia-reperfusion injury in mouse brain and heart. In the myocardial ischemia-reperfusion injury (MIRI) model, pretreatment with GFGs at 2.5 ml/kg was superior to the same dose of GGs in improving cardiac function and coronary blood flow and reducing the levels of lactate dehydrogenase and aspartate aminotransferase in serum, with an effect similar to that achieved by SXNI. In contrast, pretreatment with GGs at 2.5 ml/kg reduced cerebral infarction area and cerebral edema similarly to that of SXNI but more significantly compared with GFGs in cerebral ischemia-reperfusion injury (CIRI) model. Network pharmacology analysis of GFGs and GGs revealed that tumor necrosis factor-related weak inducer of apoptosis (TWEAK)-fibroblast growth factor-inducible 14 (Fn14) signaling pathway as an important common mechanism but with differential targets in MIRI and CIRI. In addition, immunohistochemistry and enzyme linked immunosorbent assay (ELISA) assays were performed to evaluate the regulatory roles of GFGs and GGs on the common TWEAK-Fn14 signaling pathway to protect the heart and brain. Experimental results confirmed that TWEAK ligand and Fn14 receptor were downregulated by GFGs to mitigate MIRI in the heart while upregulated by GGs to improve CIRI in the brain. In conclusion, our study showed that GFGs and GGs of SXNI tend to differentially protect brain and heart from ischemia-reperfusion injuries at least in part by regulating a common TWEAK-Fn14 signaling pathway.
Keywords: Shuxuening injection; TWEAK–Fn14 signaling; cerebral ischemia—reperfusion injury; ginkgo flavonol glycosides; ginkgolides; myocardial ischemia—reperfusion injury.
Figures

References
-
- Bederson J. B., Pitts L. H., Germano S. M., Nishimura M. C., Davis R. L., Bartkowski H. M. (1986). Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 17 (6), 1304–1308. 10.1161/01.STR.17.6.1304 - DOI - PubMed
LinkOut - more resources
Full Text Sources

