Nrf2 Plays a Protective Role Against Intravascular Hemolysis-Mediated Acute Kidney Injury
- PMID: 31333462
- PMCID: PMC6619398
- DOI: 10.3389/fphar.2019.00740
Nrf2 Plays a Protective Role Against Intravascular Hemolysis-Mediated Acute Kidney Injury
Abstract
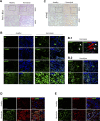
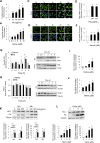
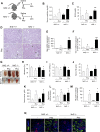
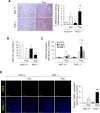
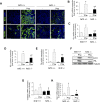
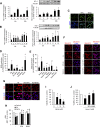
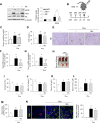
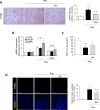
Massive intravascular hemolysis is associated with acute kidney injury (AKI). Nuclear factor erythroid-2-related factor 2 (Nrf2) plays a central role in the defense against oxidative stress by activating the expression of antioxidant proteins. We investigated the role of Nrf2 in intravascular hemolysis and whether Nrf2 activation protected against hemoglobin (Hb)/heme-mediated renal damage in vivo and in vitro. We observed renal Nrf2 activation in human hemolysis and in an experimental model of intravascular hemolysis promoted by phenylhydrazine intraperitoneal injection. In wild-type mice, Hb/heme released from intravascular hemolysis promoted AKI, resulting in decreased renal function, enhanced expression of tubular injury markers (KIM-1 and NGAL), oxidative and endoplasmic reticulum stress (ER), and cell death. These features were more severe in Nrf2-deficient mice, which showed decreased expression of Nrf2-related antioxidant enzymes, including heme oxygenase 1 (HO-1) and ferritin. Nrf2 activation with sulforaphane protected against Hb toxicity in mice and cultured tubular epithelial cells, ameliorating renal function and kidney injury and reducing cell stress and death. Nrf2 genotype or sulforaphane treatment did not influence the severity of hemolysis. In conclusion, our study identifies Nrf2 as a key molecule involved in protection against renal damage associated with hemolysis and opens novel therapeutic approaches to prevent renal damage in patients with severe hemolytic crisis. These findings provide new insights into novel aspects of Hb-mediated renal toxicity and may have important therapeutic implications for intravascular hemolysis-related diseases.
Keywords: Nrf2; heme; hemoglobin; intravascular hemolysis; oxidative stress; sulforaphane; tubular injury.
Figures
References
-
- Balla G., Jacob H. S., Balla J., Rosenberg M., Nath K., Apple F., et al. (1992). Ferritin: a cytoprotective antioxidant strategem of endothelium. J. Biol. Chem. 267 (25), 18148–18153. - PubMed
-
- Ballarin J., Arce Y., Torra Balcells R., Diaz Encarnacion M., Manzarbeitia F., Ortiz A., et al. (2011). Acute renal failure associated to paroxysmal nocturnal haemoglobinuria leads to intratubular haemosiderin accumulation and CD163 expression. Nephrol. Dial. Transplant. 26 (10), 3408–3411. 10.1093/ndt/gfr391 - DOI - PubMed

