Identification of a PEST Sequence in Vertebrate KIR2.1 That Modifies Rectification
- PMID: 31333502
- PMCID: PMC6624654
- DOI: 10.3389/fphys.2019.00863
Identification of a PEST Sequence in Vertebrate KIR2.1 That Modifies Rectification
Abstract
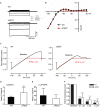
KIR2.1 potassium channels, producing inward rectifier potassium current (I K1 ), are important for final action potential repolarization and a stable resting membrane potential in excitable cells like cardiomyocytes. Abnormal KIR2.1 function, either decreased or increased, associates with diseases such as Andersen-Tawil syndrome, long and short QT syndromes. KIR2.1 ion channel protein trafficking and subcellular anchoring depends on intrinsic specific short amino acid sequences. We hypothesized that combining an evolutionary based sequence comparison and bioinformatics will identify new functional domains within the C-terminus of the KIR2.1 protein, which function could be determined by mutation analysis. We determined PEST domain signatures, rich in proline (P), glutamic acid (E), serine (S), and threonine (T), within KIR2.1 sequences using the "epestfind" webtool. WT and ΔPEST KIR2.1 channels were expressed in HEK293T and COS-7 cells. Patch-clamp electrophysiology measurements were performed in the inside-out mode on excised membrane patches and the whole cell mode using AxonPatch 200B amplifiers. KIR2.1 protein expression levels were determined by western blot analysis. Immunofluorescence microscopy was used to determine KIR2.1 subcellular localization. An evolutionary conserved PEST domain was identified in the C-terminus of the KIR2.1 channel protein displaying positive PEST scores in vertebrates ranging from fish to human. No similar PEST domain was detected in KIR2.2, KIR2.3, and KIR2.6 proteins. Deletion of the PEST domain in California kingsnake and human KIR2.1 proteins (ΔPEST), did not affect plasma membrane localization. Co-expression of WT and ΔPEST KIR2.1 proteins resulted in heterotetrameric channel formation. Deletion of the PEST domain did not increase protein stability in cycloheximide assays [T½ from 2.64 h (WT) to 1.67 h (ΔPEST), n.s.]. WT and ΔPEST channels, either from human or snake, produced typical I K1 , however, human ΔPEST channels displayed stronger intrinsic rectification. The current observations suggest that the PEST sequence of KIR2.1 is not associated with rapid protein degradation, and has a role in the rectification behavior of I K1 channels.
Keywords: KIR2.1; PEST domain; channel; inward rectifier; patch clamp; potassium; vertebrates.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

