miR-489-3p Regulates the Oxidative Stress Response in the Liver and Gill Tissues of Hybrid Yellow Catfish (Pelteobagrus fulvidraco♀ × P. vachelli♂) Under Cu2+ Exposure by Targeting Cu/Zn-SOD
- PMID: 31333503
- PMCID: PMC6624672
- DOI: 10.3389/fphys.2019.00868
miR-489-3p Regulates the Oxidative Stress Response in the Liver and Gill Tissues of Hybrid Yellow Catfish (Pelteobagrus fulvidraco♀ × P. vachelli♂) Under Cu2+ Exposure by Targeting Cu/Zn-SOD
Abstract
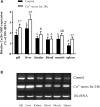
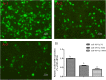
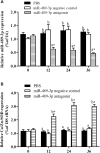
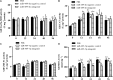
Copper/zinc superoxide dismutase (Cu/Zn-SOD) plays critical roles in protecting cells and tissues against oxidative damage. Excessive copper ions (Cu2+) in water can damage the cells of aquatic organisms, leading to impaired growth and development and reduced antioxidant defenses. Many regulatory factors control the response to excess Cu2+. Among them, microRNAs (miRNAs) are important small RNAs that regulate the expression of their target genes and participate in the oxidative stress response. In the present study, we used bioinformatics and dual luciferase reporter gene analyses to demonstrate that the miR-489-3p of hybrid yellow catfish (Pelteobagrus fulvidraco♀ × P. vachelli♂) binds to the 3'-untranslated region (UTR) of its target gene, which encodes a Cu/Zn-SOD. The regulatory relationship between this miRNA and its target gene Cu/Zn-SOD was analyzed using qRT-PCR and luciferase activity assays. We also investigated the effect of the loss of miR-489-3p expression on the oxidative stress response of hybrid yellow catfish exposed to Cu2+. The Cu/Zn-SOD 3'UTR region was found to be fully complementary to positions 2-9 of the 5'-end seed region of miR-489-3p. The miR-489-3p expression levels were negatively related to Cu/Zn-SOD expression. Silencing of miR-489-3p up-regulated Cu/Zn-SOD expression in the liver and gill tissues, increased activities of SOD and catalase, and reduced the malondialdehyde content. This study is the first to demonstrate that miR-489-3p targets Cu/Zn-SOD to mediate the oxidative response to metal stress. These findings provide a theoretical basis for further studies on the response to oxidative stress caused by metals in cultured fish, and provide an experimental basis for the management of the culture environment.
Keywords: Cu/Zn-SOD; Cu2+; antioxidant system; hybrid yellow catfish (Pelteobagrus fulvidraco♀ × P. vachelli♂); miR-489-3p.
Figures



Similar articles
-
Responses of functional miRNA-mRNA regulatory modules to a high-fat diet in the liver of hybrid yellow catfish (Pelteobagrus fulvidraco × P. vachelli).Genomics. 2021 Jan;113(1 Pt 2):1207-1220. doi: 10.1016/j.ygeno.2020.12.007. Epub 2020 Dec 9. Genomics. 2021. PMID: 33309769
-
The effects of temperature and dissolved oxygen on the growth, survival and oxidative capacity of newly hatched hybrid yellow catfish larvae (Tachysurus fulvidraco♀ × Pseudobagrus vachellii♂).J Therm Biol. 2019 Dec;86:102436. doi: 10.1016/j.jtherbio.2019.102436. Epub 2019 Oct 10. J Therm Biol. 2019. PMID: 31789232
-
MiR-205 Mediated Cu-Induced Lipid Accumulation in Yellow Catfish Pelteobagrus fulvidraco.Int J Mol Sci. 2018 Sep 29;19(10):2980. doi: 10.3390/ijms19102980. Int J Mol Sci. 2018. PMID: 30274304 Free PMC article.
-
Integrated miRNA and mRNA Sequencing Reveals the Sterility Mechanism in Hybrid Yellow Catfish Resulting from Pelteobagrus fulvidraco (♀) × Pelteobagrus vachelli (♂).Animals (Basel). 2024 May 27;14(11):1586. doi: 10.3390/ani14111586. Animals (Basel). 2024. PMID: 38891632 Free PMC article.
-
Characterization of two copper/zinc superoxide dismutases (Cu/Zn-SODs) from the desert beetle Microdera punctipennis and their activities in protecting E. coli cells against cold.Cryobiology. 2019 Apr;87:15-27. doi: 10.1016/j.cryobiol.2019.03.006. Epub 2019 Mar 16. Cryobiology. 2019. PMID: 30890324 Review.
Cited by
-
Nuclear and Morphological Alterations in Erythrocytes, Antioxidant Enzymes, and Genetic Disparities Induced by Brackish Water in Mrigal Carp (Cirrhinus mrigala).Oxid Med Cell Longev. 2022 Oct 11;2022:4972622. doi: 10.1155/2022/4972622. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36267815 Free PMC article.
-
Effects of acute hypoxia and reoxygenation on oxygen sensors, respiratory metabolism, oxidative stress, and apoptosis in hybrid yellow catfish "Huangyou-1".Fish Physiol Biochem. 2021 Oct;47(5):1429-1448. doi: 10.1007/s10695-021-00989-8. Epub 2021 Jul 27. Fish Physiol Biochem. 2021. PMID: 34313912
-
Effects of Dietary Protein-to-Energy Ratios on Growth, Immune Response, Antioxidative Capacity, Liver and Intestinal Histology, and Growth-Related Gene Expression in Hybrid Yellow Catfish (Pelteobagrus fulvidraco ♀ × Pelteobagrus vachelli ♂).Aquac Nutr. 2023 May 23;2023:9106332. doi: 10.1155/2023/9106332. eCollection 2023. Aquac Nutr. 2023. PMID: 37260466 Free PMC article.
-
Selenium-Cultured Potamogeton maackianus in the Diet Can Alleviate Oxidative Stress and Immune Suppression in Chinese Mitten Crab (Eriocheir sinensis) Under Copper Exposure.Front Physiol. 2020 Jun 19;11:713. doi: 10.3389/fphys.2020.00713. eCollection 2020. Front Physiol. 2020. PMID: 32655418 Free PMC article.
-
Adipose Mesenchymal Stem Cell-Derived Exosomes Promote the Regeneration of Corneal Endothelium Through Ameliorating Senescence.Invest Ophthalmol Vis Sci. 2023 Oct 3;64(13):29. doi: 10.1167/iovs.64.13.29. Invest Ophthalmol Vis Sci. 2023. PMID: 37850944 Free PMC article.
References
-
- Alrumman S. A., El-kott A. F., Kehsk M. A. (2016). Water pollution: source and treatment. Am. J. Environ. Eng. 6 88–98.
-
- Baldissera M. D., Souza C. F., Parmeggiani B., Leipnitz G., Verdi C. M., Santos R., et al. (2018). The disturbance of antioxidant/oxidant balance in fish experimentally infected by Aeromonas caviae: relationship with disease pathophysiology. Microb. Pathogenesis 122 53–57. 10.1016/j.micpath.2018.06.011 - DOI - PubMed
-
- Chakravarthy N., Kalyanasundaram A., Kalaimani N., Alavandi S. V., Poornima M., Santiago T. C. (2012). Intracellular Copper Zinc Superoxide dismutase (icCuZnSOD) from Asian seabass (Lates calcarifer): molecular cloning, characterization and gene expression with reference to Vibrio anguillarum infection. Dev. comp. immunol. 36 751–755. 10.1016/j.dci.2011.11.002 - DOI - PubMed
LinkOut - more resources
Full Text Sources

