Potential Negative Effects of Dextromethorphan as an Add-On Therapy to Methylphenidate in Children With ADHD
- PMID: 31333511
- PMCID: PMC6620613
- DOI: 10.3389/fpsyt.2019.00437
Potential Negative Effects of Dextromethorphan as an Add-On Therapy to Methylphenidate in Children With ADHD
Abstract
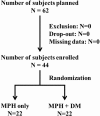
Objectives: Methylphenidate (MPH) is highly effective in controlling the symptoms of attention-deficit/hyperactivity disorder (ADHD), but some children with ADHD either do not respond to, or do not tolerate, treatment. Dextromethorphan (DM) is a neuroprotective agent which has been used in the treatment of neuropsychiatric disorders. This clinical trial had examined the effect of DM on the use of MPH in the children with ADHD. Methods: This randomized double-blind clinical trial had evaluated 44 male outpatients, aged between 6 and 12 years, with a diagnosis of ADHD. The study subjects were randomly assigned into one of the two groups: receiving MPH alone (15-60 mg per day) or MPH plus DM (30-60 mg per day) for 8 weeks. Assessments, comprising the Chinese version of the Child Behavior Checklist (CBCL-C) scale and the Swanson, Nolan and Pelham Questionnaire (SNAP)-IV rating tests conducted by parents and the serum cytokines measured by microarray and enzyme-linked immunosorband assay (ELISA), were compared between groups at baseline and at 8 weeks after the medication was started. Results: There were a significant decrease at the mean scores of both CBCL-C and SNAP-IV scales after 8 weeks of treatment, but no significant differences between MPH and MPH+DM groups. Compared with the MPH-only group, the mean scores of some psychometric parameters reported on the CBCL-C and SNAP-IV scales regarding time effects as well as the attention problems on the CBCL-C scale regarding group effect were significantly higher in the DM+MPH group. Although there were no significant differences in the levels of various serum cytokines between groups, the subjects in the DM-MPH group had relatively fewer and lower levels of adverse effects. Significant interactions were found between the withdrawn/depression item reported on the CBCL-C scale and tumor necrosis factor α (ခTNF-α) (p = 0.027), as well as between thought problems item on the CBCL-C and TNF-α (p = 0.028) in subjects who had received DM+MPH treatment. Conclusion: Following the trial, DM+MPH was not superior to MPH alone for the treatment of children with ADHD, yet DM may potentially have negative effects on ADHD symptoms when combined with MPH. Clinical Trial Registration: Clinicaltrials.gov, trial number: NCT01787136.
Keywords: ADHD; added-on therapy; children; cytokines; dextromethorphan; methylphenidate.
Similar articles
-
Efficacy and Safety of a Chewable Methylphenidate Extended-Release Tablet in Children with Attention-Deficit/Hyperactivity Disorder.J Child Adolesc Psychopharmacol. 2017 Oct;27(8):690-699. doi: 10.1089/cap.2016.0177. Epub 2017 May 30. J Child Adolesc Psychopharmacol. 2017. PMID: 28557548 Free PMC article. Clinical Trial.
-
A randomized crossover clinical study showing that methylphenidate-SODAS improves attention-deficit/hyperactivity disorder symptoms in adolescents with substance use disorder.Braz J Med Biol Res. 2008 Mar;41(3):250-7. doi: 10.1590/s0100-879x2008005000011. Epub 2008 Feb 20. Braz J Med Biol Res. 2008. PMID: 18327433 Clinical Trial.
-
A double-blind, placebo-controlled trial of dexmethylphenidate hydrochloride and d,l-threo-methylphenidate hydrochloride in children with attention-deficit/hyperactivity disorder.J Am Acad Child Adolesc Psychiatry. 2004 Nov;43(11):1406-14. doi: 10.1097/01.chi.0000138351.98604.92. J Am Acad Child Adolesc Psychiatry. 2004. PMID: 15502600 Clinical Trial.
-
Randomized, Double-Blind, Placebo-Controlled Acute Comparator Trials of Lisdexamfetamine and Extended-Release Methylphenidate in Adolescents With Attention-Deficit/Hyperactivity Disorder.CNS Drugs. 2017 Nov;31(11):999-1014. doi: 10.1007/s40263-017-0468-2. CNS Drugs. 2017. PMID: 28980198 Free PMC article.
-
Does chirality matter? pharmacodynamics of enantiomers of methylphenidate in patients with attention-deficit/hyperactivity disorder.J Clin Psychopharmacol. 2008 Jun;28(3 Suppl 2):S62-6. doi: 10.1097/JCP.0b013e3181744aa6. J Clin Psychopharmacol. 2008. PMID: 18480679 Review.
Cited by
-
A Practical, Evidence-informed Approach to Managing Stimulant-Refractory Attention Deficit Hyperactivity Disorder (ADHD).CNS Drugs. 2021 Oct;35(10):1035-1051. doi: 10.1007/s40263-021-00848-3. Epub 2021 Aug 17. CNS Drugs. 2021. PMID: 34403134 Review.
References
-
- Lasky-Su J, Neale BM, Franke B, Anney RJ, Zhou K, Maller JB, et al. Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. Am J Med Genet B Neuropsychiatr Genet (2008) 147B(8):1345–54. 10.1002/ajmg.b.30867 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical