International Guidelines for the Treatment of Huntington's Disease
- PMID: 31333565
- PMCID: PMC6618900
- DOI: 10.3389/fneur.2019.00710
International Guidelines for the Treatment of Huntington's Disease
Abstract
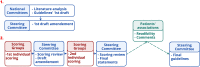
The European Huntington's Disease Network (EHDN) commissioned an international task force to provide global evidence-based recommendations for everyday clinical practice for treatment of Huntington's disease (HD). The objectives of such guidelines are to standardize pharmacological, surgical and non-pharmacological treatment regimen and improve care and quality of life of patients. A formalized consensus method, adapted from the French Health Authority recommendations was used. First, national committees (French and English Experts) reviewed all studies published between 1965 and 2015 included dealing with HD symptoms classified in motor, cognitive, psychiatric, and somatic categories. Quality grades were attributed to these studies based on levels of scientific evidence. Provisional recommendations were formulated based on the strength and the accumulation of scientific evidence available. When evidence was not available, recommendations were framed based on professional agreement. A European Steering committee supervised the writing of the final recommendations through a consensus process involving two rounds of online questionnaire completion with international multidisciplinary HD health professionals. Patients' associations were invited to review the guidelines including the HD symptoms. Two hundred and nineteen statements were retained in the final guidelines. We suggest to use this adapted method associating evidence base-medicine and expert consensus to other rare diseases.
Keywords: Huntington's disease; care; clinical practice; guidelines; treatment.
References
-
- Armstrong MJ, Miyasaki JM. American Academy of Neurology. Evidence-based guideline: pharmacologic treatment of chorea in Huntington disease: report of the guideline development subcommittee of the American Academy of Neurology. Neurology. (2012) 79:597–603. 10.1212/WNL.0b013e318263c443 - DOI - PMC - PubMed