Dosage Related Efficacy and Tolerability of Cannabidiol in Children With Treatment-Resistant Epileptic Encephalopathy: Preliminary Results of the CARE-E Study
- PMID: 31333569
- PMCID: PMC6616248
- DOI: 10.3389/fneur.2019.00716
Dosage Related Efficacy and Tolerability of Cannabidiol in Children With Treatment-Resistant Epileptic Encephalopathy: Preliminary Results of the CARE-E Study
Abstract
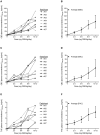
Purpose: There is uncertainty regarding the appropriate dose of Cannabidiol (CBD) for childhood epilepsy. We present the preliminary data of seven participants from the Cannabidiol in Children with Refractory Epileptic Encephalopathy (CARE-E) study. Methods: The study is an open-label, prospective, dose-escalation trial. Participants received escalating doses of a Cannabis Herbal Extract (CHE) preparation of 1:20 Δ9-tetrahydrocannabinol (THC): CBD up to 10-12 mg CBD/kg/day. Seizure frequency was monitored in daily logs, participants underwent regular electroencephalograms, and parents filled out modified Quality of Life in Childhood Epilepsy (QOLCE) and Side Effect rating scale questionnaires. Steady-state trough levels (Css, Min) of selected cannabinoids were quantified. Results: All seven participants tolerated the CHE up to 10-12 mg CBD/kg/day and had improvements in seizure frequency and QOLCE scores. CSS, Min plasma levels for CBD, THC, and cannabichromene (CBC) showed dose-independent pharmacokinetics in all but one participant. CSS, Min CBD levels associated with a >50% reduction in seizures and seizure freedom were lower than those reported previously with purified CBD. In most patients, CSS, Min levels of THC remained lower than what would be expected to cause intoxication. Conclusion: The preliminary data suggest an initial CBD target dose of 5-6 mg/kg/day when a 1:20 THC:CBD CHE is used. Possible non-linear pharmacokinetics of CBD and CBC needs investigation. The reduction in seizure frequency seen suggests improved seizure control when a whole plant CHE is used. Plasma THC levels suggest a low risk of THC intoxication when a 1:20 THC:CBD CHE is used in doses up to 12 mg/kg CBD/kg/day.
Keywords: cannabidiol; cannabinoid plasma levels; cannabis; epileptic encephalopathy; Δ9-tetrahydrocannabinol.
Figures
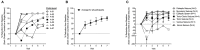

References
-
- Thiele EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome(GWPCARE4); a randomized, double blind, placebo controlled phase 3 trial. Lancet. (2018) 391:1085–96. 10.1016/S0140-6736(18)30136-3 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

