Raw Material Regulates Flavor Formation via Driving Microbiota in Chinese Liquor Fermentation
- PMID: 31333623
- PMCID: PMC6620735
- DOI: 10.3389/fmicb.2019.01520
Raw Material Regulates Flavor Formation via Driving Microbiota in Chinese Liquor Fermentation
Abstract
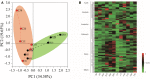
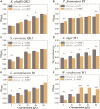
Raw material is important for flavors in fermented foods. Here, the effect of hulless barley on the microbiota in Chinese liquor was studied using two main cultivars (heilaoya and dulihuang). Six genera (Lactobacillus, Saccharomyces, Komagataella, Aspergillus, Pichia, and Weissella) were identified as flavor producers. Komagataella, mainly correlated with esters, dominated in heilaoya, and Pichia, mainly correlated with carbonyls, dominated in dulihuang. The Mantel test indicated reducing sugar drove the succession of microbiota (heilaoya: P = 0.001; dulihuang: P = 0.006). Especially, glucose (P = 0.0226) and fructose (P = 0.0168) presented the most significant correlations with Pichia and Komagataella, respectively. The simulative fermentation confirmed Komagataella phaffii QK2 grew better in heilaoya with more fructose, whereas Pichia fermentans PF grew better in dulihuang with more glucose. This work highlighted the effect of raw material on microbiota, which would be beneficial for regulating the quality of fermented foods.
Keywords: Chinese liquor; flavor producers; fructose; glucose; hulless barley.
Figures





Similar articles
-
Construction of Synthetic Microbiota for Reproducible Flavor Compound Metabolism in Chinese Light-Aroma-Type Liquor Produced by Solid-State Fermentation.Appl Environ Microbiol. 2019 May 2;85(10):e03090-18. doi: 10.1128/AEM.03090-18. Print 2019 May 15. Appl Environ Microbiol. 2019. PMID: 30850432 Free PMC article.
-
Environmental Microbiota Drives Microbial Succession and Metabolic Profiles during Chinese Liquor Fermentation.Appl Environ Microbiol. 2018 Jan 31;84(4):e02369-17. doi: 10.1128/AEM.02369-17. Print 2018 Feb 15. Appl Environ Microbiol. 2018. PMID: 29196296 Free PMC article.
-
Dynamics of quality attributes, flavor compounds, and microbial communities during multi-driven-levels chili fermentation: Interactions between the metabolome and microbiome.Food Chem. 2023 Mar 30;405(Pt B):134936. doi: 10.1016/j.foodchem.2022.134936. Epub 2022 Nov 12. Food Chem. 2023. PMID: 36435118
-
Initial fungal diversity impacts flavor compounds formation in the spontaneous fermentation of Chinese liquor.Food Res Int. 2022 May;155:110995. doi: 10.1016/j.foodres.2022.110995. Epub 2022 Feb 25. Food Res Int. 2022. PMID: 35400416
-
Research Progress on Flavor Compounds and Microorganisms of Maotai Flavor Baijiu.J Food Sci. 2019 Jan;84(1):6-18. doi: 10.1111/1750-3841.14409. Epub 2018 Dec 12. J Food Sci. 2019. PMID: 30548499 Review.
Cited by
-
Unraveling the regional environmental ecology dominated baijiu fermentation microbial community succession and associated unique flavor.Front Microbiol. 2024 Oct 31;15:1487359. doi: 10.3389/fmicb.2024.1487359. eCollection 2024. Front Microbiol. 2024. PMID: 39545237 Free PMC article. Review.
-
Amino Acids Drive the Deterministic Assembly Process of Fungal Community and Affect the Flavor Metabolites in Baijiu Fermentation.Microbiol Spectr. 2023 Mar 21;11(2):e0264022. doi: 10.1128/spectrum.02640-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36943039 Free PMC article.
-
Effects of ultra-long fermentation time on the microbial community and flavor components of light-flavor Xiaoqu Baijiu based on fermentation tanks.World J Microbiol Biotechnol. 2021 Nov 24;38(1):3. doi: 10.1007/s11274-021-03183-3. World J Microbiol Biotechnol. 2021. PMID: 34817705 Free PMC article.
-
High-throughput sequencing of the microbial diversity of roasted-sesame-like flavored Daqu with different characteristics.3 Biotech. 2020 Nov;10(11):502. doi: 10.1007/s13205-020-02500-1. Epub 2020 Nov 2. 3 Biotech. 2020. PMID: 33163321 Free PMC article.
-
Deciphering the Shifts in Microbial Community Diversity From Material Pretreatment to Saccharification Process of Fuyu-Flavor Baijiu.Front Microbiol. 2021 Aug 20;12:705967. doi: 10.3389/fmicb.2021.705967. eCollection 2021. Front Microbiol. 2021. PMID: 34489894 Free PMC article.
References
LinkOut - more resources
Full Text Sources

