A Novel Method for Identification and Quantification of Sulfated Flavonoids in Plants by Neutral Loss Scan Mass Spectrometry
- PMID: 31333712
- PMCID: PMC6625178
- DOI: 10.3389/fpls.2019.00885
A Novel Method for Identification and Quantification of Sulfated Flavonoids in Plants by Neutral Loss Scan Mass Spectrometry
Abstract
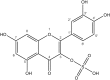
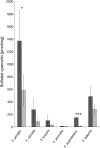
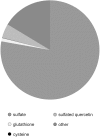
Sulfur is present in plants in a large range of essential primary metabolites, as well as in numerous natural products. Many of these secondary metabolites contain sulfur in the oxidized form of organic sulfate. However, except of glucosinolates, very little is known about other classes of such sulfated metabolites, mainly because of lack of specific and quantitative analytical methods. We developed an LC-MS method to analyze sulfated flavonoids, a group of sulfated secondary metabolites prominent, e.g., in plants of the genus Flaveria. The method uses a linear gradient of methanol/formic acid in water on a Restek Raptor C18 Core-Shell column for separation of the compounds. The sulfated flavonoids are detected by mass spectrometry (MS) in a negative mode, using a neutral loss of 80 Da after a collision induced dissociation. With this method we were also able to quantify the sulfated flavonoids. We could detect all (mono)sulfated flavonoids described before in Flaveria plus a number of new ones, such as isorhamnetin-sulfate-glycoside. In addition, we showed that sulfated flavonoids represent a substantial sulfur pool in Flaveria, larger than the thiols glutathione and cysteine. The individual species possess different sulfated flavonoids, but there is no correlation between the qualitative pattern and type of photosynthesis. Similar to other sulfur-containing secondary compounds, the concentration of sulfated flavonoids in leaves is reduced by sulfur starvation. The new LC-MS method will enable qualitative and quantitative detection of these secondary metabolites in plants as a pre-requisite to addressing their functions.
Keywords: Flaveria; mass spectrometry; method development; neutral loss scan; sulfated flavonoids; sulfur metabolism.
Figures




Similar articles
-
Control of sulfur partitioning between primary and secondary metabolism.Plant J. 2011 Jan;65(1):96-105. doi: 10.1111/j.1365-313X.2010.04410.x. Epub 2010 Nov 22. Plant J. 2011. PMID: 21175893
-
Quantitative determination of flavonoids by column high-performance liquid chromatography with mass spectrometry and ultraviolet absorption detection in Artemisia afra and comparative studies with various species of Artemisia plants.J AOAC Int. 2009 Mar-Apr;92(2):633-44. J AOAC Int. 2009. PMID: 19485225
-
Rapid screening method for intact glucosinolates in Chinese medicinal herbs by using liquid chromatography coupled with electrospray ionization ion trap mass spectrometry in negative ion mode.Rapid Commun Mass Spectrom. 2008 Sep;22(18):2825-34. doi: 10.1002/rcm.3669. Rapid Commun Mass Spectrom. 2008. PMID: 18711760
-
Emerging sulfated flavonoids and other polyphenols as drugs: nature as an inspiration.Med Res Rev. 2014 Mar;34(2):223-79. doi: 10.1002/med.21282. Epub 2013 Mar 28. Med Res Rev. 2014. PMID: 23553315 Review.
-
Manipulation of thiol contents in plants.Amino Acids. 2001;20(3):291-9. doi: 10.1007/s007260170045. Amino Acids. 2001. PMID: 11354605 Review.
Cited by
-
Sulfation pathways from red to green.J Biol Chem. 2019 Aug 16;294(33):12293-12312. doi: 10.1074/jbc.REV119.007422. Epub 2019 Jul 2. J Biol Chem. 2019. PMID: 31270211 Free PMC article. Review.
-
Improved Chrysin Production by a Combination of Fermentation Factors and Elicitation from Chaetomium globosum.Microorganisms. 2023 Apr 12;11(4):999. doi: 10.3390/microorganisms11040999. Microorganisms. 2023. PMID: 37110422 Free PMC article.
-
Bioherbicidal Activity and Metabolic Profiling of Allelopathic Metabolites of Three Cassia species using UPLC-qTOF-MS/MS and Molecular Networking.Metabolomics. 2023 Mar 9;19(3):16. doi: 10.1007/s11306-023-01980-5. Metabolomics. 2023. PMID: 36892715
-
Insight into chemical basis of traditional Chinese medicine based on the state-of-the-art techniques of liquid chromatography-mass spectrometry.Acta Pharm Sin B. 2021 Jun;11(6):1469-1492. doi: 10.1016/j.apsb.2021.02.017. Epub 2021 Feb 26. Acta Pharm Sin B. 2021. PMID: 34221863 Free PMC article. Review.
-
Comment on Tremmel et al. In Vitro Metabolism of Six C-Glycosidic Flavonoids from Passiflora incarnata L. Int. J. Mol. Sci. 2021, 22, 6566.Int J Mol Sci. 2022 Apr 18;23(8):4445. doi: 10.3390/ijms23084445. Int J Mol Sci. 2022. PMID: 35457262 Free PMC article.
References
-
- Amr A., Al-Tamimi E. (2007). Stability of the crude extracts of Ranunculus asiaticus anthocyanins and their use as food colourants. Int. J. Food Sci. Technol. 42 985–991. 10.1111/j.1365-2621.2006.01334.x - DOI
LinkOut - more resources
Full Text Sources

