Pharmacological agents for adults with acute respiratory distress syndrome
- PMID: 31334568
- PMCID: PMC6646953
- DOI: 10.1002/14651858.CD004477.pub3
Pharmacological agents for adults with acute respiratory distress syndrome
Abstract
Background: Acute respiratory distress syndrome (ARDS) is a life-threatening condition caused by direct or indirect injury to the lungs. Despite improvements in clinical management (for example, lung protection strategies), mortality in this patient group is at approximately 40%. This is an update of a previous version of this review, last published in 2004.
Objectives: To evaluate the effectiveness of pharmacological agents in adults with ARDS on mortality, mechanical ventilation, and fitness to return to work at 12 months.
Search methods: We searched CENTRAL, MEDLINE, Embase, and CINAHL on 10 December 2018. We searched clinical trials registers and grey literature, and handsearched reference lists of included studies and related reviews.
Selection criteria: We included randomized controlled trials (RCTs) comparing pharmacological agents with control (placebo or standard therapy) to treat adults with established ARDS. We excluded trials of nitric oxide, inhaled prostacyclins, partial liquid ventilation, neuromuscular blocking agents, fluid and nutritional interventions and medical oxygen. We excluded studies published earlier than 2000, because of changes to lung protection strategies for people with ARDS since this date.
Data collection and analysis: Two review authors independently assessed studies for inclusion, extracted data, and assessed risks of bias. We assessed the certainty of evidence with GRADE.
Main results: We included 48 RCTs with 6299 participants who had ARDS; two included only participants with mild ARDS (also called acute lung injury). Most studies included causes of ARDS that were both direct and indirect injuries. We noted differences between studies, for example the time of administration or the size of dose, and because of unclear reporting we were uncertain whether all studies had used equivalent lung protection strategies.We included five types of agents as the primary comparisons in the review: corticosteroids, surfactants, N-acetylcysteine, statins, and beta-agonists. We included 15 additional agents (sivelestat, mesenchymal stem cells, ulinastatin, anisodimine, angiotensin-converting enzyme (ACE) inhibitor, recombinant human ACE2 (palifermin), AP301, granulocyte-macrophage colony stimulating factor (GM-CSF), levosimendan, prostacyclins, lisofylline, ketaconazole, nitroglycerins, L-2-oxothiazolidine-4-carboxylic acid (OTZ), and penehyclidine hydrochloride).We used GRADE to downgrade outcomes for imprecision (because of few studies and few participants), for study limitations (e.g. high risks of bias) and for inconsistency (e.g. differences between study data).Corticosteroids versus placebo or standard therapyCorticosteroids may reduce all-cause mortality within three months by 86 per 1000 patients (with as many as 161 fewer to 19 more deaths); however, the 95% confidence interval (CI) includes the possibility of both increased and reduced deaths (risk ratio (RR) 0.77, 95% CI 0.57 to 1.05; 6 studies, 574 participants; low-certainty evidence). Due to the very low-certainty evidence, we are uncertain whether corticosteroids make little or no difference to late all-cause mortality (later than three months) (RR 0.99, 95% CI 0.64 to 1.52; 1 study, 180 participants), or to the duration of mechanical ventilation (mean difference (MD) -4.30, 95% CI -9.72 to 1.12; 3 studies, 277 participants). We found that ventilator-free days up to day 28 (VFD) may be improved with corticosteroids (MD 4.09, 95% CI 1.74 to 6.44; 4 studies, 494 participants; low-certainty evidence). No studies reported adverse events leading to discontinuation of study medication, or fitness to return to work at 12 months (FTR).Surfactants versus placebo or standard therapyWe are uncertain whether surfactants make little or no difference to early mortality (RR 1.08, 95% CI 0.91 to 1.29; 9 studies, 1338 participants), or whether they reduce late all-cause mortality (RR 1.28, 95% CI 1.01 to 1.61; 1 study, 418 participants). Similarly, we are uncertain whether surfactants reduce the duration of mechanical ventilation (MD -2.50, 95% CI -4.95 to -0.05; 1 study, 16 participants), make little or no difference to VFD (MD -0.39, 95% CI -2.49 to 1.72; 2 studies, 344 participants), or to adverse events leading to discontinuation of study medication (RR 0.50, 95% CI 0.17 to 1.44; 2 studies, 88 participants). We are uncertain of these effects because we assessed them as very low-certainty. No studies reported FTR.N-aceytylcysteine versus placeboWe are uncertain whether N-acetylcysteine makes little or no difference to early mortality, because we assessed this as very low-certainty evidence (RR 0.64, 95% CI 0.32 to 1.30; 1 study, 36 participants). No studies reported late all-cause mortality, duration of mechanical ventilation, VFD, adverse events leading to study drug discontinuation, or FTR.Statins versus placeboStatins probably make little or no difference to early mortality (RR 0.99, 95% CI 0.78 to 1.26; 3 studies, 1344 participants; moderate-certainty evidence) or to VFD (MD 0.40, 95% CI -0.71 to 1.52; 3 studies, 1342 participants; moderate-certainty evidence). Statins may make little or no difference to duration of mechanical ventilation (MD 2.70, 95% CI -3.55 to 8.95; 1 study, 60 participants; low-certainty evidence). We could not include data for adverse events leading to study drug discontinuation in one study because it was unclearly reported. No studies reported late all-cause mortality or FTR.Beta-agonists versus placebo controlBeta-blockers probably slightly increase early mortality by 40 per 1000 patients (with as many as 119 more or 25 fewer deaths); however, the 95% CI includes the possibility of an increase as well as a reduction in mortality (RR 1.14, 95% CI 0.91 to 1.42; 3 studies, 646 participants; moderate-certainty evidence). Due to the very low-certainty evidence, we are uncertain whether beta-agonists increase VFD (MD -2.20, 95% CI -3.68 to -0.71; 3 studies, 646 participants), or make little or no difference to adverse events leading to study drug discontinuation (one study reported little or no difference between groups, and one study reported more events in the beta-agonist group). No studies reported late all-cause mortality, duration of mechanical ventilation, or FTR.
Authors' conclusions: We found insufficient evidence to determine with certainty whether corticosteroids, surfactants, N-acetylcysteine, statins, or beta-agonists were effective at reducing mortality in people with ARDS, or duration of mechanical ventilation, or increasing ventilator-free days. Three studies awaiting classification may alter the conclusions of this review. As the potential long-term consequences of ARDS are important to survivors, future research should incorporate a longer follow-up to measure the impacts on quality of life.
Conflict of interest statement
Sharon R Lewis: none known Michael W Pritchard: none known Carmel M Thomas: none known Andrew F Smith: none known
Figures
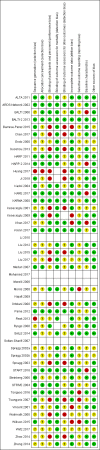









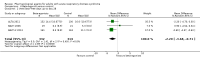





Update of
-
Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome.Cochrane Database Syst Rev. 2004 Oct 18;2004(4):CD004477. doi: 10.1002/14651858.CD004477.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2019 Jul 23;7:CD004477. doi: 10.1002/14651858.CD004477.pub3. PMID: 15495113 Free PMC article. Updated.
References
References to studies included in this review
ALTA 2011 {published data only}
ARDS Network 2002 {published data only}
-
- The ARDS Clinical Trials Network, National Heart, Lung and Blood Institute, National Institutes of Health. Randomized, placebo‐controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Critical Care Medicine 2002;30(1):1‐6. [PUBMED: 11902249] - PubMed
BALTI 2006 {published data only}
-
- Gao F, Thickett DR. In vivo and in vitro effects of salbutamol on alveolar epithelial repair in acute lung injury. Thorax 2008;63(3):215‐20. [PUBMED: 17951278] - PubMed
-
- Perkins GD, McAuley DF, Thickett DR, Gao F. The ß‐agonist lung injury trial (BALTI). American Journal of Respiratory and Critical Care Medicine 2006;173(3):281‐7. [PUBMED: 16254268] - PubMed
BALTI‐2 2013 {published data only}
-
- Gao Smith F, Perkins GD, Gates S, Young D, McAuley DF, Tunnicliffe W, et al. Effect of intravenous beta‐2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI‐2): a multicentre, randomised controlled trial. Lancet 2012;379(9812):229‐35. [PUBMED: 22166903] - PMC - PubMed
-
- Gates S, Perkins GD, Lamb SE, Kelly C, Thickett DR, Young JD, et al. Beta‐agonist lung injury trIal‐2 (BALTI‐2): a multicentre, randomised, double‐blind, placebo‐controlled trial and economic evaluation of intravenous infusion of salbutamol versus placebo in patients with acute respiratory distress syndrome. Health Technology Assessment 2013;17(38):v‐vi: 1‐87. [PUBMED: 24028755] - PMC - PubMed
Barrese‐Perez 2015 {published data only}
-
- Barrese‐Perez Y, Hidalgo‐Sanchez AO, Avila‐Albuerne Y, Uranga‐Pina R, Diaz‐Casanas E, Fernandez‐Limia O. Pulmonary surfactant exogenous in adults with acute respiratory distress syndrome. Revista del Instituto Nacional de Enfermedades Respiratorias 2015;74(3):176‐8.
Chen 2017 {published data only}
-
- Chen Y, Luo JJ, Jiang DX, Lin YP, Tong HS, Su L. Effect of ulinastatin on acute lung injury induced by severe heat stroke and its mechanism. Medical Journal of Chinese People's Liberation Army 2017;42(4):301‐6. [Embase: 616874253]
Endo 2006 {published data only}
-
- Endo S, Sato N, Yaegashi Y, Suzuki Y, Kojika M, Yamada Y, et al. Sivelestat sodium hydrate improves septic acute lung injury by reducing alveolar dysfunction. Research Communications in Molecular Pathology and Pharmacology 2006;119(1‐6):53‐65. [PUBMED: 17974096] - PubMed
Guoshou 2013 {published data only}
-
- Guoshou Z, Chengye Z, Zhihui L, Jinlong L. Effects of high dose of anisodamine on the respiratory function of patients with traumatic acute lung injury. Cell Biochemistry and Biophysics 2013;66(2):365‐9. [PUBMED: 23504631] - PubMed
HARP 2011 {published data only}
-
- Brealey D, Hargreaves I, Land J, Singer M, McAuley D. Plasma ubiquinone levels are unaffected by simvastatin in acute lung injury. Intensive Care Medicine 2012;38:S64.
-
- Craig TR, Duffy MJ, Shyamsundar M, McDowell C, O'Kane CM, Elborn JS, et al. A randomized clinical trial of hydroxymethylglutaryl‐coenzyme a reductase inhibition for acute lung injury (the HARP study). American Journal of Respiratory and Critical Care Medicine 2011;183(5):620‐6. [PUBMED: 20870757] - PubMed
-
- Craig TR, Duffy MJ, Shyamsundar M, McDowell C, O'Kane CM, Elborn JS, et al. Erratum: a randomized clinical trial of hydroxymethylglutaryl‐coenzyme a reductase inhibition for acute lung injury (the HARP study). American Journal of Respiratory and Critical Care Medicine 2014;190(10):1199‐200. - PubMed
-
- Craig TR, Duffy MJ, Shyamsundar M, O'Kane C, Elborn JS, McAuley DF. Simvastatin reduces inflammation and improves clinical outcomes in ALI: results of The HARP Study. Thorax 2009;64:A2.
-
- Craig TR, Duffy MJ, Shyamsundar M, O'Kane CM, Elborn JS, McAuley DF. Results of the HARP study: a randomized double blind phase II trial of 80mg simvastatin in acute lung injury. American Journal of Respiratory and Critical Care Medicine. Conference: American Thoracic Society International Conference, ATS. 2010; Vol. 181:no pagination.
HARP‐2 2014 {published data only}
-
- Laffey JG, O'Kane CM, Cross M, Perkins GD, Murphy L, McNally C, et al. Hydroxymethylglutaryl‐CoA reductase inhibition with simvastatin in acute lung injury to reduce pulmonary dysfunction (HARP‐2) trial: study protocol for a randomized controlled trial. Trials 2012;13(170):1‐9. [PUBMED: 22985805] - PMC - PubMed
-
- McAuley DF, Laffey JG, O'Kane CM, Perkins GD, Mullan B, Trinder T, et al. Simvastatin in the acute respiratory distress syndrome. New England Journal of Medicine 2014;371(18):1695‐703. [PUBMED: 25268516] - PubMed
-
- McAuley DF, Laffey JG, O'Kane CM, Perkins GD, Mullan B, Trinder TJ, et al. SImvastatin to reduce pulmonary dysfunction in patients with acute respiratory distress syndrome: the HARP‐2 RCT. Efficacy and Mechanism Evaluation 2018;5(1):no pagination. [PUBMED: 29400921] - PubMed
Huang 2017 {published data only}
Ji 2018 {published data only}
Kadoi 2004 {published data only}
-
- Kadoi Y, Hinohara H, Kunimoto F, Saito S, Goto F, Kosaka T, et al. Pilot study of the effects of ONO‐5046 in patients with acute respiratory distress syndrome. Anesthesia and Analgesia 2004;99(3):872‐7. [PUBMED: 15333424] - PubMed
KARE 2017 {published data only}
-
- McAuley DF, Cross LJ, Hamid U, Gardner E, Elborn JS, Cullen KM, et al. Keratinocyte growth factor for the treatment of the acute respiratory distress syndrome (KARE): a randomised, double‐blind, placebo‐controlled phase 2 trial. Lancet Respiratory Medicine 2017;5(6):484‐91. [PUBMED: 28526233] - PubMed
KARMA 2000 {published data only}
-
- National Heart Lung and Blood Institute, The ARDS Clinical Trials Network. Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2000;283(15):1995‐2002. [PUBMED: 10789668] - PubMed
Kesecioglu 2001 {published data only}
-
- Kesecioglu J, Schultz MJ, Lundberg D, Lauven PM, Lachmann B. Treatment of acute lung injury (ALI/ARDS) with surfactant. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Pt. 2):A819.
Kesecioglu 2009 {published data only}
-
- Kesecioglu J, Beale R, Stewart TE, Findlay GP, Rouby JJ, Holzapfel L, et al. Exogenous natural surfactant for treatment of acute lung injury and the acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 2009;180(10):989‐94. [PUBMED: 19713451] - PubMed
Khan 2017 {published data only}
Krenn 2017 {published data only}
-
- Krenn K, Lucas R, Croizé A, Boehme S, Klein KU, Hermann R, et al. Inhaled AP301 for treatment of pulmonary edema in mechanically ventilated patients with acute respiratory distress syndrome: a phase IIa randomized placebo‐controlled trial. Critical Care 2017;21(1):194. [PUBMED: 28750677] - PMC - PubMed
Li 2010 {published data only}
-
- Li BQ, Sun HC, Nie SN, Shao DB, Liu HM, Qian XM. Effect of penehyclidine hydrochloride on patients with acute lung injury and its mechanisms. Chinese Journal of Traumatology ‐ English Edition 2010;13(6):329‐35. [PUBMED: 21126389] - PubMed
Liu 2012 {published data only}
-
- Liu L, Li J, Huang YZ, Liu SQ, Yang CS, Guo FM, et al. The effect of stress dose glucocorticoid on patients with acute respiratory distress syndrome combined with critical illness‐related corticosteroid insufficiency. Chinese Journal of Internal Medicine 2012;51(8):599‐603. [PUBMED: 23158856] - PubMed
Liu 2015 {published data only}
-
- Liu LP, Li B, Zhu L, Dou ZM, Li YM. Clinical efficacy of nitroglycerin in patients with septic shock with ARDS. Medical Journal of Chinese People's Liberation Army 2015;40(8):647‐51. [EMBASE: 2015326178]
Liu 2017 {published data only}
-
- Liu LP, Hu SW, Shuai TK, Deng YY, Wang LJ, Li YM. Clinical effect of alprostadil in patients with septic shock associated with acute respiratory distress syndrome. Medical Journal of Chinese People's Liberation Army 2017;42(9):805‐9. [Embase: 20170777094]
Meduri 2007 {published data only}
-
- Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, et al. Methylprednisolone infusion in early severe ARDS results of a randomized controlled trial. Chest 2009;136(5 Suppl):e30. [Embase: 358384513] - PubMed
-
- Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest 2007;131(4):954‐63. [PUBMED: 17426195] - PubMed
Mohamed 2017 {published data only}
Morelli 2006 {published data only}
-
- Morelli A, Teboul JL, Maggiore SM, Vieillard‐Baron A, Rocco M, Conti G, et al. Effects of levosimendan on right ventricular afterload in patients with acute respiratory distress syndrome: a pilot study. Critical Care Medicine 2006;34(9):2287‐93. [PUBMED: 16791109] - PubMed
Morris 2008 {published data only}
-
- Morris PE, Papadakos P, Russell JA, Wunderink R, Schuster DP, Truwit JD, et al. A double‐blind placebo‐controlled study to evaluate the safety and efficacy of L‐2‐oxothiazolidine‐4‐carboxylic acid in the treatment of patients with acute respiratory distress syndrome. Critical Care Medicine 2008;36(3):782‐8. [PUBMED: 18209670] - PubMed
Najafi 2009 {published data only}
-
- Najafi A, Mojtahedzadeh M, Mahmoodpoor A, Aghamohammadi M, Ahmadi A, Nahreini S, et al. Effect of N‐acetylcysteine on microalbuminuria in patients with acute respiratory distress syndrome. Archives of Medical Science 2009;5(3):408‐4. [EMBASE: 358052253]
Ortolani 2000 {published data only}
-
- Ortolani O, Conti A, Gaudio AR, Masoni M, Novelli G. Protective effects of N‐acetylcysteine and rutin on the lipid peroxidation of the lung epithelium during the adult respiratory distress syndrome. Shock 2000;13(1):14‐8. [PUBMED: 10638663] - PubMed
Paine 2012 {published data only}
-
- Coley C, Dechert R, Harding S, Paine R, Moss M, Martin GS, et al. Granulocyte macrophage colony stimulating factor is associated with decreased quality of life at 180 days post‐event when administered to patients with acute lung injury. American Journal of Respiratory and Critical Care Medicine. Conference: American Thoracic Society International Conference, ATS 2012;185:no pagination. [Embase: 71986815]
Rezk 2013 {published data only}
-
- Rezk NA, Ibrahim AM. Effects of methyl prednisolone in early ARDS. Egyptian Journal of Chest Diseases and Tuberculosis 2013;62(1):167‐72. [EMBASE: 369315254]
Ryugo 2006 {published data only}
-
- Ryugo M, Sawa Y, Takano H, Matsumiya G, Iwai S, Ono M, et al. Effect of a polymorphonuclear elastase inhibitor (sivelestat sodium) on acute lung injury after cardiopulmonary bypass: findings of a double‐blind randomized study. Surgery Today 2006;36(4):321‐6. [PUBMED: 16554988] - PubMed
SAILS 2014 {published data only}
-
- Needham DM, Colantuoni E, Dinglas VD, Hough CL, Wozniak AW, Jackson JC, et al. Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis‐associated acute respiratory distress syndrome: an ancillary study to a randomised controlled trial. Lancet Respiratory Medicine 2016;4(3):203‐12. [PUBMED: 26832963] - PMC - PubMed
-
- Rogers A, Guan J, Hunninghake GM, Trtchounian A, Liu K, Matthay MA, et al. Inflammasome activation is associated with ARDS mortality, particularly in patients randomized to statin therapy. American Journal of Respiratory and Critical Care Medicine 2017;195:no pagination. [Embase: 617705951]
-
- Truwit JD, Bernard GR, Steingrub J, Matthay MA, Liu K, Albertson TE, et al. Sails: Statins for acutely injured lungs (ARDS) from sepsis. American Journal of Respiratory and Critical Care Medicine. Conference: American Thoracic Society International Conference, ATS 2014;189:no pagination. [Embase: 72047035]
Soltan‐Sharifi 2007 {published data only}
-
- Soltan‐Sharifi MS, Mojtahedzadeh M, Najafi A, Reza Khajavi M, Reza Rouini M, Moradi M, et al. Improvement by N‐acetylcysteine of acute respiratory distress syndrome through increasing intracellular glutathione, and extracellular thiol molecules and anti‐oxidant power: evidence for underlying toxicological mechanisms. Human and Experimental Toxicology 2007;26(9):697‐703. [PUBMED: 17984140] - PubMed
Spragg 2002a {published data only}
-
- Spragg RG, Lewis JF, Rathgeb F, Hafner D, Seeger W. Intratracheal instillation of rSP‐C surfactant improves oxygenation in patients with ARDS. American Journal of Respiratory and Critical Care Medicine 2002;165(5 Pt. 2):A22.
Spragg 2002b {published data only}
-
- Spragg RG, Lewis JF, Rathgeb F, Hafner D, Seeger W. Intratracheal instillation of rSP‐C surfactant improves oxygenation in patients with ARDS. American Journal of Respiratory and Critical Care Medicine 2002;165(5 Pt. 2):A22.
Spragg 2003 {published data only}
-
- Spragg RG, Lewis JF, Wurst W, Häfner D, Baughman RP, Wewers MD. Treatment of acute respiratory distress syndrome with recombinant surfactant protein C surfactant. American Journal of Respiratory and Critical Care Medicine 2003;185(1):1562‐6. [PUBMED: 12649125] - PubMed
START 2018 {published data only}
-
- Liu KD, Wilson JG, Zhuo H, Caballero L, McMillan ML, Fang X, et al. Design and implementation of the START (stem cells for ARDS treatment) trial, a phase 1/2 trial of human mesenchymal stem/stromal cells for the treatment of moderate‐severe acute respiratory distress syndrome. Annals of Intensive Care 2014;4(1):1‐9. [PUBMED: 25593740] - PMC - PubMed
-
- Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respiratory Medicine 2019; Vol. 7, issue 2:154‐62. [PUBMED: 30455077] - PMC - PubMed
Steinberg 2006 {published data only}
-
- Meduri GU, Bridges L, Siemieniuk RA, Kocak M. An exploratory reanalysis of the randomized trial on efficacy of corticosteroids as rescue therapy for the late phase of acute respiratory distress syndrome. Critical Care Medicine 2018;46(6):884‐91. [PUBMED: 29432350] - PubMed
-
- Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. New England Journal of Medicine 2006;354(16):1671‐84. [PUBMED: 16625008] - PubMed
STRIVE 2004 {published data only}
-
- Zeiher BG, Artigas A, Vincent JL, Dmitrienko A, Jackson K, Thompson BT, et al. Neutrophil elastase inhibition in acute lung injury: results of the STRIVE study. Critical Care Medicine 2004;32(8):1695‐702. [PUBMED: 15286546] - PubMed
Tongyoo 2016 {published data only}
-
- Tongyoo S, Permpikul C, Vattanavanit V, Mongkolpun W. Hydrocortisone in treatment of severe sepsis and septic shock with acute respiratory distress syndrome: a randomised controlled trial. Intensive Care Medicine Experimental 2015;3:no pagination. [Embase: 611853166]
Tsangaris 2007 {published data only}
-
- Tsangaris I, Galiatsou E, Kostanti E, Nakos G. The effect of exogenous surfactant in patients with lung contusions and acute lung injury. Intensive Care Medicine 2007;33(5):851‐5. [PUBMED: 17377767] - PubMed
Vincent 2001 {published data only}
-
- Vincent JL, Brase R, Santman F, Suter PM, McLuckie A, Dhainaut JF, et al. A multi‐centre, double‐blind, placebo‐controlled study of liposomal prostaglandin E1 (TLC C‐53) in patients with acute respiratory distress syndrome. Intensive Care Medicine 2001;27(10):1578‐83. [PUBMED: 11685297] - PubMed
Walmrath 2000 {published data only}
-
- Walmrath D, Vaal JB, Bruining HA, Kilian JG, Papazian L, Hohlfeld J, et al. Treatment of ARDS with a recombinant SP‐C (rSP‐C) based synthetic surfactant. American Journal of Respiratory and Critical Care Medicine 2000;161:A379.
Willson 2015 {published data only}
-
- Willson DF, Truwit JD, Conaway MR, Traul CS, Egan EE. The adult calfactant in acute respiratory distress syndrome trial. Chest 2015;148(2):356‐64. [PUBMED: 25855884] - PubMed
Wirtz 2017 {published data only}
-
- Wirtz H, Hasenclever D, Schwabe K, Jaschinski U, Weyland A, Kuhnt E, et al. ACE inhibitor for lung protection during mechanical ventilation for acute lung injury‐results of the double‐blind, placebo controlled, randomised ACEmeVent pilot study. American Journal of Respiratory and Critical Care Medicine. Conference: American Thoracic Society International Conference, ATS 2017. United states 2017;195:no pagination. [Embase: 617707751]
Zhao 2014 {published data only}
-
- Zhao WB, Wan SX, Gu DF, Shi B. Therapeutic effect of glucocorticoid inhalation for pulmonary fibrosis in ARDS patients. Medical Journal of Chinese People's Liberation Army 2014;39(9):741‐5. [EMBASE: 2014842327]
Zheng 2014 {published data only}
-
- Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose‐derived mesenchymal stem cells: a randomized, placebo‐controlled pilot study. American Journal of Respiratory and Critical Care Medicine. Conference: American Thoracic Society International Conference, ATS 2014;189:no pagination. - PMC - PubMed
References to studies excluded from this review
Abraham 1996 {published data only}
-
- Abraham E, Park YC, Covington P, Conrad SA, Schwartz M. Liposomal prostaglandin E1 in acute respiratory distress syndrome: a placebo‐controlled, randomized, double‐blind, multicenter clinical trial. Critical Care Medicine 1996;24(1):10‐5. [PUBMED: 8565513] - PubMed
Abraham 1999 {published data only}
-
- Abraham E, Baughman R, Fletcher E, Heard S, Lamberti J, Levy H, et al. Liposomal prostaglandin E1 (TLC C‐53) in acute respiratory distress syndrome: a controlled, randomized, double‐blind, multicenter clinical trial. TLC C‐53 ARDS Study Group. Critical Care Medicine 1999;27(8):1478‐85. [PUBMED: 10470753] - PubMed
Annane 2006 {published data only}
-
- Annane D, Sebille V, Bellissant E. Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Critical Care Medicine 2006;34(1):22‐30. [PUBMED: 16374152] - PubMed
Anzueto 1996 {published data only}
-
- Anzueto A, Baughman RP, Guntupalli KK, Weg JG, Wiedemann HP, Raventos AA, et al. Aerosolized surfactant in adults with sepsis‐induced acute respiratory distress syndrome. Exosurf Acute Respiratory Distress Syndrome Sepsis Study Group. New England Journal of Medicine 1996;334(22):1417‐21. [PUBMED: 8618579] - PubMed
Ardizzoia 1993 {published data only}
-
- Ardizzoia A, Lissoni P, Tancini G, Paolorossi F, Crispino S, Villa S, et al. Respiratory distress syndrome in patients with advanced cancer treated with pentoxifylline: a randomized study. Support Care in Cancer 1993;1(6):331‐3. [PUBMED: 8156252] - PubMed
Bastin 2010 {published data only}
-
- Bastin AJ, Lagan AL, Mumby S, Quinlan GJ, Griffiths MJD. Effect of N‐acetylcysteine in preventing inflammation after lung resection and one‐lung ventilation. A randomised controlled trial. American Journal of Respiratory and Critical Care Medicine 2010;181(1):no pagination.
Bastin 2016 {published data only}
-
- Bastin AJ, Davies N, Lim E, Quinlan GJ, Griffiths MJ. Systemic inflammation and oxidative stress post‐lung resection: Effect of pretreatment with N‐acetylcysteine. Respirology 2016;21(1):180‐7. [PUBMED: 26503312] - PubMed
Bernard 1987 {published data only}
-
- Bernard GR, Luce JM, Sprung CL, Rinaldo JE, Tate RM, Sibbald WJ, et al. High‐dose corticosteroids in patients with the adult respiratory distress syndrome. New England Journal of Medicine 1987;317(25):1565‐70. [PUBMED: 3317054] - PubMed
Bernard 1997 {published data only}
-
- Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, et al. A trial of antioxidants N‐acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest 1997;112(1):164‐72. [PUBMED: 9228372] - PubMed
Bernard 1999 {published data only}
-
- Bernard GR, Wheeler AP, Naum CC, Morris PE, Nelson L, Schein R, et al. A placebo‐controlled, randomized trial of IL‐10 in acute lung injury (ALI). Chest. 1999; Vol. 116, issue 4 Suppl 2:206S.
Bone 1989 {published data only}
-
- Bone RC, Slotman G, Maunder R, Silverman H, Hyers TM, Kerstein MD, et al. Randomized double‐blind, multicenter study of prostaglandin E1 in patients with the adult respiratory distress syndrome. Prostaglandin E1 Study Group. Chest 1989;96(1):114‐9. [PUBMED: 2661155] - PubMed
-
- Slotman GJ, Kerstein MD, Bone RC, Silverman H, Maunder R, Hyers TM, et al. The effects of prostaglandin E1 on non‐pulmonary organ function during clinical acute respiratory failure. The Prostaglandin E1 Study Group. Journal of Trauma 1992;32(4):480‐8. [PUBMED: 1569622] - PubMed
Confalonieri 2005 {published data only}
-
- Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, et al. Hydrocortisone infusion for severe community‐acquired pneumonia: a preliminary randomized study. American Journal of Respiratory and Critical Care Medicine 2005;171(3):242‐8. [PUBMED: 15557131] - PubMed
Cornet 2014 {published data only}
Domenighetti 1997 {published data only}
-
- Domenighetti G, Suter PM, Schaller MD, Ritz R, Perret C. Treatment with N‐acetylcysteine during acute respiratory distress syndrome: a randomized, double‐blind, placebo‐controlled clinical study. Journal of Critical Care 1997;12(4):177‐82. [PUBMED: 9459113] - PubMed
Forel 2006 {published data only}
-
- Forel JM, Roch A, Marin V, Michelet P, Demory D, Blache JL, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Critical Care Medicine 2006;34(11):2749‐57. [PUBMED: 16932229] - PubMed
Gainnier 2004 {published data only}
-
- Gainnier M, Roch A, Forel JM, Thirion X, Arnal JM, Donati S, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Critical Care Medicine 2004;32(1):113‐9. [PUBMED: 14707568] - PubMed
Gottlieb 1994 {published data only}
-
- Gottlieb JE, Elmer M, Steiner R. Efficacy of intravenous ICI 200,880, a neutrophil elastase inhibitor, in the treatment of adult respiratory distress syndrome (ARDS). Chest 1994;106(Suppl 2):67S.
Gregory 1997 {published data only}
-
- Gregory TJ, Steinberg KP, Spragg R, Gadek JE, Hyers TM, Longmore WJ, et al. Bovine surfactant therapy for patients with acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 1997;155(4):1309‐15. [PUBMED: 9105072] - PubMed
Holcroft 1986 {published data only}
Hua 2013 {published data only}
Jepsen 1992 {published data only}
-
- Jepsen S, Herlevsen P, Knudsen P, Bud MI, Klausen NO. Antioxidant treatment with N‐acetylcysteine during adult respiratory distress syndrome: a prospective, randomized, placebo‐controlled study. Critical Care Medicine 1992;20(7):918‐23. [PUBMED: 1617983] - PubMed
Liu 2008 {published data only}
Markart 2007 {published data only}
Meduri 1998 {published data only}
-
- Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA 1998;280(2):159‐65. [PUBMED: 9669790] - PubMed
Papazian 2010 {published data only}
-
- Papazian L, Forel JM, Gacouin A, Penot‐Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. New England Journal of Medicine 2010;363(12):1107‐16. [PUBMED: 20843245] - PubMed
-
- Papazian L, Forel JM, Gacouin A, Perrin G, Jaber S, Arnal JM, et al. Systematic two‐day muscle relaxants course in the early phase of severe acute respiratory distress syndrome. A multicenter randomized controlled trial. Intensive Care Medicine 2009;35:S6. [Embase: 70190221]
-
- Papazian L, Forel JM, Morange S, Barrau K, Penot‐Ragon C. Interest of a 48 h systematic paralysis at the early phase, in patients presenting with ARDS. The ACURASYS study. Protocol presentation [Interet d'une curarisation systematique precoce de 48 heures sur le pronostic des patients presentant un syndrome de detresse respiratoire aigue. Etude ACURASYS. Presentation du protocole]. Reanimation 2007;16(1):88‐95.
Presneill 2002 {published data only}
-
- Presneill JJ, Harris T, Stewart AG, Cade JF, Wilson JW. A randomized phase II trial of granulocyte‐macrophage colony‐stimulating factor therapy in severe sepsis with respiratory dysfunction. American Journal of Respiratory and Critical Care Medicine 2002;166(2):138‐43. [PUBMED: 12119223] - PubMed
Reines 1985 {published data only}
-
- Reines HD, Halushka PV, Olanoff LS, Hunt PS. Dazoxiben in human sepsis and adult respiratory distress syndrome. Clinical Pharmacology and Therapeutics 1985;37(4):391‐5. [PUBMED: 3979000] - PubMed
Reines 1992 {published data only}
-
- Reines HD, Sliverman H, Hurst J, Warren J, Williams J, Rotello L, et al. Effects of two concentrations of nebulized surfactant (Exosurf) in sepsis‐induced adult respiratory distress syndrome (ARDS). Critical Care Medicine 1992;20(4 Suppl):S61.
-
- Wiedermann H, Baughman R, DeBoisblanc B, Schuster D, Caldwell E, Weg J, et al. A multicenter trial in human sepsis‐induced ARDS of an aerosolized synthetic surfactant (Exosurf). American Review of Respiratory Disease 1992;145(4 part 2):A184.
Rossignon 1990 {published data only}
-
- Rossignon MD, Khayat D, Royer C, Rouby JJ, Jacquillat C, Viars P. Functional and metabolic activity of polymorphonuclear leukocytes from patients with adult respiratory distress syndrome: results of a randomized double‐blind placebo‐controlled study on the activity of prostaglandin E1. Anesthesiology 1990;72(2):276‐81. [PUBMED: 2405741] - PubMed
Shoemaker 1986 {published data only}
-
- Shoemaker WC. Effectiveness of prostaglandin E1 in adult respiratory distress syndrome. Progress in Clinical and Biological Research 1987;236A:361‐81. [PUBMED: 3615440] - PubMed
-
- Shoemaker WC, Appel PL. Effects of prostaglandin E1 in adult respiratory distress syndrome. Surgery 1986;99(3):275‐83. [PUBMED: 3513357] - PubMed
Shyamsundar 2010 {published data only}
-
- Shyamsundar M, O'Kane CM, Calfee C, McKeown ST, Taggart C, Matthay MA, et al. KGF enhances pulmonary production of proepithelial repair factors in a human in vivo model of acute lung injur y. Thorax 2010;65:A47. [Embase: 70325792]
Steinberg 1990 {published data only}
-
- Steinberg SM, Rodriguez JL, Bitzer LG, Rhee JW, Kelley KA, Flint LM. Indomethacin treatment of human adult respiratory distress syndrome. Circulatory Shock 1990;30(4):375‐84. [PUBMED: 2350875] - PubMed
Suter 1994 {published data only}
-
- Suter PM, Domenighetti G, Schaller MD, Laverriere MC, Ritz R, Perret C. N‐acetylcysteine enhances recovery from acute lung injury in man. A randomized, double‐blind, placebo‐controlled clinical study. Chest 1994;105(1):190‐4. [PUBMED: 8272731] - PubMed
Tuxen 1987 {published data only}
-
- Tuxen DV, Wilson JW, Cade JF. Prevention of lower respiratory herpes simplex virus infection with acyclovir in patients with the adult respiratory distress syndrome. American Review of Respiratory Distress 1987;136(2):402‐5. [PUBMED: 3039882] - PubMed
Vincent 2009 {published data only}
-
- Vincent JL, Artigas A, Petersen LC, Meyer C. A multicenter, randomized, double‐blind, placebo‐controlled, dose‐escalation trial assessing safety and efficacy of active site inactivated recombinant factor VIIa in subjects with acute lung injury or acute respiratory distress syndrome. Critical Care Medicine 2009;37(6):1874‐80. [PUBMED: 19384216] - PubMed
Weg 1994 {published data only}
-
- Doig C, Weg JG. Aerosolized surfactant in sepsis‐induced adult respiratory distress syndrome. JAMA 1995;273(16):1259‐60. [PUBMED: 7715036] - PubMed
-
- Weg JG, Balk RA, Tharratt RS, Jenkinson SG, Shah JB, Zaccardelli D, et al. Safety and potential efficacy of an aerosolized surfactant in human sepsis‐induced adult respiratory distress syndrome. JAMA 1994;272(18):1433‐8. [PUBMED: 7933425] - PubMed
Weigelt 1985 {published data only}
-
- Weigelt JA, Norcross JF, Borman KR, Snyder WH 3rd. Early steroid therapy for respiratory failure. Archives of Surgery 1985;120(5):536‐40. [PUBMED: 3885915] - PubMed
References to studies awaiting assessment
Hegazy 2016 {published data only}
-
- Hegazy S, Helmy T, Salah M. Role of nebivolol therapy in patients with severe acute respiratory distress syndrome. Critical Care Medicine 2016;44(12 Supplement 1):314.
NCT00879606 {published data only}
-
- NCT00879606. Efficacy and safety evaluation of ALT‐836 in patients with sepsis and acute lung injury/acute respiratory distress syndrome. www.clinicaltrials.gov/ct2/show/NCT00879606 (first received 10 April 2009).
RPCEC00000126 {published data only}
-
- RPCEC00000126. Evaluation of the effect and safety of SURFACEN in the treatment of acute respiratory distress syndrome in adults. rpcec.sld.cu/en/trials/RPCEC00000126‐En (first received 11 April 2012).
References to ongoing studies
ACTRN12612000418875 {published data only}
-
- ACTRN12612000418875. A multi‐centre randomised, placebo controlled trial of nebulised heparin in patients with or at risk of developing acute respiratory distress syndrome, to determine if nebulised heparin improves long term physical function. www.anzctr.org.au/ACTRN12612000418875.aspx (first received 6 April 2012).
ACTRN12615000373572 {published data only}
-
- ACTRN12615000373572. Effect of nebulized budesonide on respiratory mechanics and oxygenation in patients with acute lung injury/acute respiratory distress syndrome (ALI/ARDS). www.anzctr.org.au/ACTRN12615000373572.aspx (first received 13 January 2015).
Bellingan 2017 {published data only}
-
- Bellingan G, Brealey D, Mancebo J, Mercat A, Patroniti N, Pettila V, et al. Comparison of the efficacy and safety of FP‐1201‐lyo (intravenously administered recombinant human interferon beta‐1a) and placebo in the treatment of patients with moderate or severe acute respiratory distress syndrome: study protocol for a randomized controlled trial. Trials 2017;18(1):no pagination. - PMC - PubMed
ChiCTR1800014733 {published data only}
-
- ChiCTR1800014733. Clinical study of rhGM‐CSF in the treatment of pulmonary extraneous acute respiratory distress syndrome. www.chictr.org.cn/showproj.aspx?proj=25020 (first received 31 January 2018).
ChiCTR1800014998 {published data only}
-
- ChiCTR1800014998. Human umbilical cord derived mesenchymal stem cells therapy in acute respiratory distress syndrome: a pilot study. www.chictr.org.cn/showproj.aspx?proj=25576 (first received 27 February 2018).
EUCTR2012‐000775‐17 {published data only}
-
- EudraCT2012‐000775‐17. A comparative, randomised controlled trial for evaluating the efficacy of dexamethasone administration in the treatment of patients with the acute respiratory distress syndrome. www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:201... (first received 21 November 2012).
JPRN‐JapicCTI‐163320 {published data only}
-
- JPRN‐JapicCTI‐163320. A phase 3 clinical study to evaluate the efficacy and safety of intravenous MR11A8 in the treatment of patients with moderate or severe acute respiratory distress syndrome. www.clinicaltrials.jp/user/showCteDetailE.jsp?japicId=JapicCTI‐163320 (first received 19 July 2016).
NCT02326350 {published data only}
-
- NCT02326350. Aspirin as a treatment for ARDS (STAR): a phase 2 randomised control trial. clinicaltrials.gov/ct2/show/NCT02326350 (first received 29 December 2014).
NCT02595060 {published data only}
-
- NCT02595060. GM‐CSF inhalation to improve host defense and pulmonary barrier restoration (GI‐HOPE). A randomized, double‐blind, parallel group, multicenter, phase II study. www.clinicaltrials.gov/ct2/show/NCT02595060 (first received 3 November 2015).
NCT02611609 {published data only}
-
- NCT02611609. A phase 1/2 study to assess the safety and efficacy of MultiStem® therapy in subjects with acute respiratory distress syndrome. clinicaltrials.gov/ct2/show/NCT02611609 (first received 23 November 2015).
NCT02895191 {published data only}
-
- NCT02895191. A randomized, blind, placebo‐controlled, parallel group, multi‐center study to evaluate the safety and dose response relationship of ulinastatin for acute respiratory distress syndrome (ARDS). clinicaltrials.gov/ct2/show/NCT02895191 (first received 9 September 2016).
NCT03017547 {published data only}
-
- NCT03017547. A phase 2, randomized, double‐blind, placebo‐controlled, preliminary efficacy study of IC14 in acute respiratory distress syndrome. clinicaltrials.gov/ct2/show/NCT03017547 (first received 11 January 2017).
NCT03042143 {published data only}
-
- NCT03042143. Repair of acute respiratory distress syndrome by stromal cell administration (REALIST): an open label dose escalation phase 1 trial followed by a randomized, double‐blind, placebo‐controlled phase 2 trial. clinicaltrials.gov/ct2/show/NCT03042143 (first received 3 February 2017).
NCT03202394 {published data only}
-
- NCT03202394. A phase IIa, placebo controlled, multicenter pilot study to evaluate the safety and efficacy of BIO‐11006 inhalation solution in patients with acute respiratory distress syndrome. clinicaltrials.gov/ct2/show/NCT03202394 (first received 28 June 2017).
NCT03346681 {published data only}
-
- NCT03346681. N‐acetylcysteine in early acute respiratory distress syndrome (NARDS). clinicaltrials.gov/show/NCT03346681 (first received 17 November 2017).
NCT03371498 {published data only}
-
- NCT03371498. Procollagen‐3 driven corticosteroids for persistent acute respiratory distress syndrome (ProCoCo). clinicaltrials.gov/ct2/show/nct03371498 (first received 13 December 2017).
NCT03608592 {published data only}
-
- NCT03608592. Human umbilical cord derived mesenchymal stem cells therapy in acute respiratory distress syndrome: a pilot study. clinicaltrials.gov/ct2/show/NCT03608592 (first received 1 August 2018).
Villar 2016 {published data only}
-
- NCT01731795. A comparative, randomised controlled trial for evaluating the efficacy of dexamethasone in the treatment of patients with acute respiratory distress syndrome. clinicaltrials.gov/ct2/show/NCT01731795 (first received 22 November 2012).
-
- Villar J, Belda J, Añón JM, Blanco J, Pérez‐Méndez L, Ferrando C, et al. Evaluating the efficacy of dexamethasone in the treatment of patients with persistent acute respiratory distress syndrome: Study protocol for a randomized controlled trial. Trials 2016 2016;17(342):no pagination. - PMC - PubMed
Additional references
Afshari 2017
ARDS Definition Task Force 2012
-
- The ARDS Defintion Task Force. Acute respiratory distress syndrome; the Berlin definition. JAMA 2012;307(23):2526‐33. [PUBMED: 22797452] - PubMed
ARDS Network 2000
-
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New England Journal of Medicine 2000;342(18):1301‐8. [PUBMED: 10793162] - PubMed
Ashbaugh 1967
-
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 1967;2(7511):319‐23. [PUBMED: 4143721] - PubMed
Bellani 2016
-
- Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016;315(8):788‐800. [PUBMED: 26903337] - PubMed
Bernard 1991
-
- Bernard GR. N‐acetylcysteine in experimental and clinical acute lung injury. American Journal of Medicine 1991;91(3C):54S‐9S. [PUBMED: 1928212] - PubMed
Bernard 1994
-
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American‐European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American Journal of Respiratory and Critical Care Medicine 1994;149(3 Pt 1):818‐24. [PUBMED: 7509706] - PubMed
Bloomfield 2015
Brower 2004
-
- Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al. Higher versus lower positive end‐expiratory pressures in patients with the acute respiratory distress syndrome. New England Journal of Medicine 2004;351(4):327‐36. [PUBMED: 15269312] - PubMed
Chacko 2015
-
- Chacko B, Peter JV, Tharyan P, John G, Jeyaseelan L. Pressure‐controlled versus volume‐controlled ventilation for acute respiratory failure due to acute lung injury (ALI) or acute respiratory distress syndrome (ARDS). Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD008807.pub2; PUBMED: 25586462] - DOI - PMC - PubMed
Covidence 2018 [Computer program]
-
- Veritas Health Innovation. Covidence. Version accessed 10 December 2018. Melbourne, Australia: Veritas Health Innovation.
Deeks 2017
-
- Deeks JJ, Higgins JP, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking metaanalyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Dellinger 2008
-
- Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Critical Care Medicine 2008;36(1):296‐327. [PUBMED: 18158437] - PubMed
Egger 1997
Endnote [Computer program]
-
- Thomson Reuters. Endnote X5. Philadelphia (PA): Thomson Reuters, 2011.
Feng 2018
-
- Feng Y. Efficacy of statin therapy in patients with acute respiratory distress syndrome/acute lung injury: a systematic review and meta‐analysis. European Review for Medical and Pharmacological Sciences 2018; Vol. 22, issue 10:3190‐8. [PUBMED: 29863265] - PubMed
FICM/ICS Guideline Development Group 2018
-
- The Faculty of Intensive Care Medicine and the Intensive Care Society. Guidelines on the management of acute respiratory distress syndrome. www.ics.ac.uk. Version 1 July 2018 (accessed 10 December 2018).
Galvin 2013
-
- Galvin IM, Steel A, Pinto R, Ferguson ND, Davies MW. Partial liquid ventilation for preventing death and morbidity in adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD003707.pub3; PUBMED: 23881653] - DOI - PMC - PubMed
Gao 2016
-
- Gao XQ, Li YF, Jiang ZL. Impact of statins on ALI/ARDS: a meta‐analysis. Pulmonary Pharmacology and Therapeutics 2016; Vol. 39:85‐91. [PUBMED: 27373439] - PubMed
Gebistorf 2016
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 25 January 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guay 2018
-
- Guay J, Ochroch EA, Kopp S. Intraoperative use of low volume ventilation to decrease postoperative mortality, mechanical ventilation, lengths of stay and lung injury in adults without acute lung injury. Cochrane Database of Systematic Reviews 2018, Issue 7. [DOI: 10.1002/14651858.CD011151.pub3; PUBMED: 26641378] - DOI - PMC - PubMed
Guyatt 2008
Guyatt 2011a
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence‐‐inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] - PubMed
Guyatt 2011b
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] - PubMed
Herridge 2003
-
- Herridge MS, Cheung AM, Tansey CM, Matte‐Martyn A, Diaz‐Granados N, Al Saidi F, et al Canadian Critical Care Trials Group. One‐year outcomes in survivors of the acute respiratory distress syndrome. New England Journal of Medicine 2003;348(8):683‐93. [PUBMED: 12594312] - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Hodgson 2016
-
- Hodgson C, Goligher EC, Young ME, Keating JL, Holland AE, Romero L, et al. Recruitment manoeuvres for adults with acute respiratory distress syndrome receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD006667.pub3; PUBMED: 27855477] - DOI - PMC - PubMed
Horita 2015
-
- Horita N, Hashimoto S, Miyazawa N, Fujita H, Kojima R, Inoue M, et al. Impact of corticosteroids on mortality in patients with acute respiratory distress syndrome: A systematic review and meta‐analysis. Internal Medicine 2015; Vol. 54, issue 12:1473‐9. [PUBMED: 26267908] - PubMed
Levy 2003
-
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Critical Care Medicine 2003;31(4):1250‐6. [PUBMED: 12682500] - PubMed
Luce 1998
-
- Luce JM. Acute lung injury and the acute respiratory distress syndrome. Critical Care Medicine 1998;26(2):369‐76. [PUBMED: 9468178] - PubMed
Lundstrøm 2017
-
- Lundstrøm LH, Duez CH, Nørskov AK, Rosenstock CV, Thomsen JL, Møller AM, et al. Avoidance versus use of neuromuscular blocking agents for improving conditions during tracheal intubation or direct laryngoscopy in adults and adolescents. Cochrane Database of Systematic Reviews 2017, Issue 5. [DOI: 10.1002/14651858.CD009237.pub2; PUBMED: 28513831] - DOI - PMC - PubMed
Meng 2012
-
- Meng H, Sun Y, Lu J, Fu S, Meng Z, Scott M, et al. Exogenous surfactant may improve oxygenation but not mortality in adult patients with acute lung injury/acute respiratory distress syndrome: a meta‐analysis of 9 clinical trials. Journal of Cardiothoracic & Vascular Anesthesia 2012; Vol. 26, issue 5:849‐56. [PUBMED: 22265270] - PMC - PubMed
Murray 1988
-
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. American Review of Respiratory Disease 1988;138(3):720‐3. [PUBMED: 3202424] - PubMed
Nagendran 2017
-
- Nagendran M, McAuley DF, Kruger PS, Papazian L, Truwit JD, Laffey JG, et al. Statin therapy for acute respiratory distress syndrome: an individual patient data meta‐analysis of randomised clinical trials. Intensive Care Medicine 2017; Vol. 43, issue 5:663‐71. [PUBMED: 28004129] - PubMed
Nanchal 2018
Pehora 2017
Petrucci 2013
Polderman 2018
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Santa Cruz 2013
-
- Santa Cruz R, Rojas JI, Nervi R, Heredia R, Ciapponi A. High versus low end‐expiratory pressure (PEEP) levels for mechanically ventilated adult patients with acute lung injury and acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD009098.pub2; PUBMED: 23740697] - DOI - PMC - PubMed
SCCMCMA 2006
-
- Society of Critical Care Medicine of Chinese Medical Association. Guideline of the diagnosis and treatment of ALI/ARDS. Chinese Journal of Critical Care Medicine 2006;18(12):706‐10. [PUBMED: 17166345]
Schoenfeld 2002
-
- Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator‐free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Critical Care Medicine 2002;30(8):1772‐7. [PUBMED: 12163791] - PubMed
Singh 2014
-
- Singh B, Tiwari AK, Singh K, Singh SK, Ahmed A, Erwin PJ, et al. β2 agonist for the treatment of acute lung injury: a systematic review and meta‐analysis. Respiratory Care 2014; Vol. 59, issue 2:288‐96. [23777655] - PubMed
Sterne 2017
-
- Sterne JA, Egger M, Moher D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Sud 2016
-
- Sud S, Sud M, Friedrick JO, Wunsch H, Meade MO, Ferguson ND, et al. High‐frequency oscillatory ventilation versus conventional ventilation for acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD004085.pub4; PUBMED: 27043185] - DOI - PMC - PubMed
Ware 2000
-
- Ware LB, Matthay MA. The acute respiratory distress syndrome. New England Journal of Medicine 2000;342(18):1334‐49. [PUBMED: 10793167] - PubMed
Wiedemann 2006
-
- Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. Comparison of two fluid‐management strategies in acute lung injury. New England Journal of Medicine 2006;354(24):2564‐75. [PUBMED: 16714767] - PubMed
Yang 2017
Zhang 2013
References to other published versions of this review
Adhikari 2002
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

