Roles of omental and bone marrow adipocytes in tumor biology
- PMID: 31334678
- PMCID: PMC6768257
- DOI: 10.1080/21623945.2019.1643189
Roles of omental and bone marrow adipocytes in tumor biology
Abstract
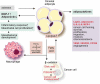
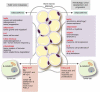
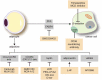
Accumulating evidence highlights the importance of interactions between tumour cells and stromal cells for tumour initiation, progression, and metastasis. In tumours that contain adipocyte in their stroma, adipocytes contribute to modification of tumour microenvironment and affect metabolism of tumour and tumour progression by production of cytokines and adipokines from the lipids. The omentum and bone marrow (BM) are highly adipocyte-rich and are also common metastatic and primary tumour developmental sites. Omental adipocytes exhibit metabolic cross-talk, immune modulation, and angiogenesis. BM adipocytes secrete adipokines, and participate in solid tumour metastasis through regulation of the CCL2/CCR2 axis and metabolic interactions. BM adipocytes also contribute to the progression of hematopoietic neoplasms. Here, we here provide an overview of research progress on the cross-talks between omental/BM adipocytes and tumour cells, which may be pivotal modulators of tumour biology, thus highlighting novel therapeutic targets. Abbreviations: MCP-1, monocyte chemoattractant protein 1IL, interleukinSTAT3, signal transducer and activator of transcription 3FABP4, fatty acid binding protein 4PI3K/AKT, phosphoinositide 3-kinase/protein kinase BPPAR, peroxisome proliferator-activated receptorPUFA, polyunsaturated fatty acidTAM, tumour-associated macrophagesVEGF, vascular endothelial growth factorVEGFR, vascular endothelial growth factor receptorBM, bone marrowBMA, bone marrow adipocytesrBMA, regulated BMAcBMA, constitutive BMAUCP-1, uncoupling protein-1TNF-α, tumour necrosis factor-alphaRANKL, receptor activator of nuclear factor kappa-Β ligandVCAM-1, vascular cell adhesion molecule 1JAK2, Janus kinase 2CXCL (C-X-C motif) ligandPGE2, prostaglandin E2COX-2, cyclooxygenase-2CCL2, C-C motif chemokine ligand 2NF-κB, nuclear factor-kappa BMM, multiple myelomaALL, acute lymphoblastic leukemiaAML, acute myeloid leukemiaGDF15, growth differentiation factor 15AMPK, AMP-activated protein kinaseMAPK, mitogen-activated protein kinaseAPL, acute promyelocytic leukemiaCCR2, C-C motif chemokine receptor 2SDF-1α, stromal cell-derived factor-1 alphaFFA, free fatty acidsLPrA, leptin peptide receptor antagonistMCD, malonyl-CoA decarboxylase.
Keywords: Bone marrow; adipokines; lipids; metastasis; omentum; tumours.
Figures
References
-
- Choi J, Cha YJ, Koo JS.. Adipocyte biology in breast cancer: From silent bystander to active facilitator. Prog Lipid Res. 2018;69:11–20. - PubMed
-
- Hoy AJ, Balaban S, Saunders DN. Adipocyte-tumor cell metabolic crosstalk in breast cancer. Trends Mol Med. 2017;23:381–392. - PubMed
-
- Wilkosz S, Ireland G, Khwaja N, et al. A comparative study of the structure of human and murine greater omentum. Anat Embryol (Berl). 2005;209:251–261. - PubMed
-
- Hall JC, Heel KA, Papadimitriou JM, et al. The pathobiology of peritonitis. Gastroenterology. 1998;114:185–196. - PubMed
-
- Goldsmith HS. Role of the omentum in the treatment of Alzheimer’s disease. Neurol Res. 2001;23:555–564. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
