Effect of Filgotinib vs Placebo on Clinical Response in Patients With Moderate to Severe Rheumatoid Arthritis Refractory to Disease-Modifying Antirheumatic Drug Therapy: The FINCH 2 Randomized Clinical Trial
- PMID: 31334793
- PMCID: PMC6652745
- DOI: 10.1001/jama.2019.9055
Effect of Filgotinib vs Placebo on Clinical Response in Patients With Moderate to Severe Rheumatoid Arthritis Refractory to Disease-Modifying Antirheumatic Drug Therapy: The FINCH 2 Randomized Clinical Trial
Erratum in
-
Incorrect Patient Numbers Reported in Tables.JAMA. 2020 Feb 4;323(5):480. doi: 10.1001/jama.2019.21742. JAMA. 2020. PMID: 32016292 Free PMC article. No abstract available.
Abstract
Importance: Patients with active rheumatoid arthritis (RA) despite treatment with biologic disease-modifying antirheumatic drug (bDMARD) therapy need treatment options.
Objective: To evaluate the effects of filgotinib vs placebo on the signs and symptoms of RA in a treatment-refractory population.
Design, setting, and participants: A 24-week, randomized, placebo-controlled, multinational phase 3 trial conducted from July 2016 to June 2018 at 114 sites internationally, randomizing 449 adult patients (and treating 448) with moderately to severely active RA and inadequate response/intolerance to 1 or more prior bDMARDs.
Interventions: Filgotinib, 200 mg (n = 148); filgotinib, 100 mg (n = 153); or placebo (n = 148) once daily; patients continued concomitant stable conventional synthetic DMARDs (csDMARDs).
Main outcomes and measures: The primary end point was the proportion of patients who achieved 20% improvement in the American College of Rheumatology criteria (ACR20) at week 12. Secondary outcomes included week 12 assessments of low disease activity (disease activity score in 28 joints-C-reactive protein [DAS28-CRP] ≤3.2) and change in Health Assessment Questionnaire-Disability Index, 36-Item Short-Form Health Survey Physical Component, and Functional Assessment of Chronic Illness Therapy-Fatigue scores, as well as week 24 assessment of remission (DAS28-CRP <2.6) and adverse events.
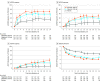
Results: Among 448 patients who were treated (mean [SD] age, 56 [12] years; 360 women [80.4%]; mean [SD] DAS28-CRP score, 5.9 [0.96]; 105 [23.4%] with ≥3 prior bDMARDs), 381 (85%) completed the study. At week 12, more patients receiving filgotinib, 200 mg (66.0%) or 100 mg (57.5%), achieved ACR20 response (placebo, 31.1%; difference vs placebo: 34.9% [95% CI, 23.5%-46.3%] and 26.4% [95% CI, 15.0%-37.9%], respectively; both P < .001), including among patients with prior exposure to 3 or more bDMARDs (70.3%, 58.8%, and 17.6%, respectively; difference vs placebo: 52.6% [95% CI, 30.3%-75.0%] for filgotinib, 200 mg, and 41.2% [95% CI, 17.3%-65.0%] for filgotinib, 100 mg; both P < .001). The most common adverse events were nasopharyngitis (10.2%) for filgotinib, 200 mg; headache, nasopharyngitis, and upper respiratory infection (5.9% each) for filgotinib, 100 mg; and RA (6.1%) for placebo. Four uncomplicated herpes zoster cases and 1 retinal vein occlusion were reported with filgotinib; there were no opportunistic infections, active tuberculosis, malignancies, gastrointestinal perforations, or deaths.
Conclusions and relevance: Among patients with active RA who had an inadequate response or intolerance to 1 or more bDMARDs, filgotinib, 100 mg daily or 200 mg daily, compared with placebo resulted in a significantly greater proportion achieving a clinical response at week 12. However, further research is needed to assess longer-term efficacy and safety.
Trial registration: ClinicalTrials.gov Identifier: NCT02873936.
Conflict of interest statement
Figures
Comment in
-
Filgotinib, a JAK1 Inhibitor, for Treatment-Resistant Rheumatoid Arthritis.JAMA. 2019 Jul 23;322(4):309-311. doi: 10.1001/jama.2019.9056. JAMA. 2019. PMID: 31334773 No abstract available.
References
-
- Walker JG, Smith MD. The Jak-STAT pathway in rheumatoid arthritis. J Rheumatol. 2005;32(9):1650-1653. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

