Near-Infrared Photoimmunotherapy of Cancer
- PMID: 31335117
- PMCID: PMC6704485
- DOI: 10.1021/acs.accounts.9b00273
Near-Infrared Photoimmunotherapy of Cancer
Abstract
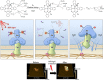
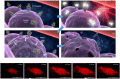
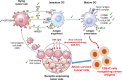
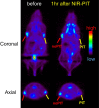
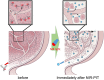
This Account is the first comprehensive review article on the newly developed, photochemistry-based cancer therapy near-infrared (NIR) photoimmunotherapy (PIT). NIR-PIT is a molecularly targeted phototherapy for cancer that is based on injecting a conjugate of a near-infrared, water-soluble, silicon-phthalocyanine derivative, IRdye700DX (IR700), and a monoclonal antibody (mAb) that targets an expressed antigen on the cancer cell surface. Subsequent local exposure to NIR light turns on this photochemical "death" switch, resulting in the rapid and highly selective immunogenic cell death (ICD) of targeted cancer cells. ICD occurs as early as 1 min after exposure to NIR light and results in irreversible morphologic changes only in target-expressing cells based on the newly discovered photoinduced ligand release reaction that induces physical changes on conjugated antibody/antigen complex resulting in functional damage on cell membrane. Meanwhile, immediately adjacent receptor-negative cells are totally unharmed. Because of its highly targeted nature, NIR-PIT carries few side effects and healing is rapid. Evaluation of the tumor microenvironment reveals that ICD induced by NIR-PIT results in rapid maturation of immature dendritic cells adjacent to dying cancer cells initiating a host anticancer immune response, resulting in repriming of polyclonal CD8+T cells against various released cancer antigens, which amplifies the therapeutic effect of NIR-PIT. NIR-PIT can target and treat virtually any cell surface antigens including cancer stem cell markers, that is, CD44 and CD133. A first-in-human phase 1/2 clinical trial of NIR-PIT using cetuximab-IR700 (RM1929) targeting EGFR in inoperable recurrent head and neck cancer patients successfully concluded in 2017 and led to "fast tracking" by the FDA and a phase 3 trial ( https://clinicaltrials.gov/ct2/show/NCT03769506 ) that is currently underway in 3 countries in Asia, US/Canada, and 4 countries in EU. The next step for NIR-PIT is to further exploit the immune response. Preclinical research in animals with intact immune systems has shown that NIT-PIT targeting of immunosuppressor cells within the tumor, such as regulatory T-cells, can further enhance tumor-cell-selective systemic host-immunity leading to significant responses in distant metastatic tumors, which are not treated with light. By combining cancer-targeting NIR-PIT and immune-activating NIR-PIT or other cancer immunotherapies, NIR-PIT of a local tumor, could lead to responses in distant metastases and may also inhibit recurrences due to activation of systemic anticancer immunity and long-term immune memory without the systemic autoimmune adverse effects often associated with immune checkpoint inhibitors. Furthermore, NIR-PIT also enhances nanodrug delivery into tumors up to 24-fold superior to untreated tumors with conventional EPR effects by intensively damaging cancer cells behind tumor vessels. We conclude by describing future advances in this novel photochemical cancer therapy that are likely to further enhance the efficacy of NIR-PIT.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Sato K.; Ando K.; Okuyama S.; Moriguchi S.; Ogura T.; Totoki S.; Hanaoka H.; Nagaya T.; Kokawa R.; Takakura H.; Nishimura M.; Hasegawa Y.; Choyke P. L.; Ogawa M.; Kobayashi H. Photoinduced Ligand Release from a Silicon Phthalocyanine Dye Conjugated with Monoclonal Antibodies: A Mechanism of Cancer Cell Cytotoxicity after Near-Infrared Photoimmunotherapy. ACS Cent. Sci. 2018, 4, 1559–1569. 10.1021/acscentsci.8b00565. - DOI - PMC - PubMed
-
- Ogawa M.; Tomita Y.; Nakamura Y.; Lee M. J.; Lee S.; Tomita S.; Nagaya T.; Sato K.; Yamauchi T.; Iwai H.; Kumar A.; Haystead T.; Shroff H.; Choyke P. L.; Trepel J. B.; Kobayashi H. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget 2017, 8, 10425–10436. 10.18632/oncotarget.14425. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

