Mitochondrial fusion exploits a therapeutic vulnerability of pancreatic cancer
- PMID: 31335325
- PMCID: PMC6777817
- DOI: 10.1172/jci.insight.126915
Mitochondrial fusion exploits a therapeutic vulnerability of pancreatic cancer
Abstract
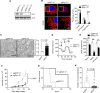
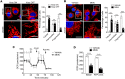
Pancreatic ductal adenocarcinoma (PDAC) requires mitochondrial oxidative phosphorylation (OXPHOS) to fuel its growth, however, broadly inhibiting this pathway might also disrupt essential mitochondrial functions in normal tissues. PDAC cells exhibit abnormally fragmented mitochondria that are essential to its oncogenicity, but it was unclear if this mitochondrial feature was a valid therapeutic target. Here, we present evidence that normalizing the fragmented mitochondria of pancreatic cancer via the process of mitochondrial fusion reduces OXPHOS, which correlates with suppressed tumor growth and improved survival in preclinical models. Mitochondrial fusion was achieved by genetic or pharmacologic inhibition of dynamin related protein-1 (Drp1) or through overexpression of mitofusin-2 (Mfn2). Notably, we found that oral leflunomide, an FDA-approved arthritis drug, promoted a two-fold increase in Mfn2 expression in tumors and was repurposed as a chemotherapeutic agent, improving the median survival of mice with spontaneous tumors by 50% compared to vehicle. We found that the chief tumor suppressive mechanism of mitochondrial fusion was enhanced mitophagy, which proportionally reduced mitochondrial mass and ATP production. These data suggest that mitochondrial fusion is a specific and druggable regulator of pancreatic cancer growth that could be rapidly translated to the clinic.
Keywords: Cancer; Gastroenterology; Mitochondria; Mouse models; Oncology.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

