A 3-month, Multicenter, Randomized, Open-label Study to Evaluate the Impact on Wound Healing of the Early (vs Delayed) Introduction of Everolimus in De Novo Kidney Transplant Recipients, With a Follow-up Evaluation at 12 Months After Transplant (NEVERWOUND Study)
- PMID: 31335776
- PMCID: PMC7004468
- DOI: 10.1097/TP.0000000000002851
A 3-month, Multicenter, Randomized, Open-label Study to Evaluate the Impact on Wound Healing of the Early (vs Delayed) Introduction of Everolimus in De Novo Kidney Transplant Recipients, With a Follow-up Evaluation at 12 Months After Transplant (NEVERWOUND Study)
Abstract
Background: The risk of wound healing complications (WHCs) and the early use of mammalian target of rapamycin inhibitors after kidney transplantation (KT) have not been fully addressed.
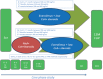
Methods: The NEVERWOUND study is a 3-month, multicenter, randomized, open-label study designed to evaluate whether a delayed (ie, 28 ± 4 d posttransplant) immunosuppression regimen based on everolimus (EVR) reduces the risk of WHC versus EVR started immediately after KT. Secondary endpoints were treatment failure (biopsy-proven acute rejection, graft loss, or death), delayed graft function, patient and graft survival rates, and renal function.
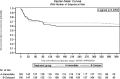
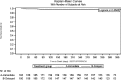
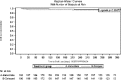
Results: Overall, 394 KT recipients were randomized to receive immediate (N = 197) or delayed (N = 197) EVR after KT. At 3 months, WHC-free rates in the immediate EVR versus delayed EVR arm, considering the worst- and best-case scenario approach, were 0.68 (95% confidence interval [CI], 0.62-0.75) versus 0.62 (95% CI, 0.55-0.68) (log-rank P = 0.56) and 0.70 (95% CI, 0.64-0.77) versus 0.72 (95% CI, 0.65-0.78) (log-rank P = 0.77), respectively. The 3- and 12-month treatment failure rates, delayed graft function and renal function, and patient and graft survival were not different between the arms.
Conclusions: The early introduction of EVR after KT did not increase the risk of WHC, showing good efficacy and safety profile.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Pascual J. Everolimus in clinical practice--renal transplantation. Nephrol Dial Transplant 200621Suppl 3iii18–iii23 - PubMed
-
- Nashan B. Induction therapy and mTOR inhibition: minimizing calcineurin inhibitor exposure in de novo renal transplant patients. Clin Transplant 201327Suppl 2516–29 - PubMed
-
- Budde K, Zeier M, Witzke O, et al. ; HERAKLES Study Group Everolimus with cyclosporine withdrawal or low-exposure cyclosporine in kidney transplantation from month 3: a multicentre, randomized trial. Nephrol Dial Transplant 2017321060–1070 - PubMed
-
- Carmellini M, Collini A, Ruggieri G, et al. Excellent long-term results in de novo renal transplant recipients treated with proliferation signal inhibitors and reduced calcineurin inhibitors exposure. Transplant Proc 2008401858–1861 - PubMed
-
- Ponticelli C, Scolari MP. Calcineurin inhibitors in renal transplantation still needed but in reduced doses: a review. Transplant Proc 2010422205–2208 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

