Immunomodulatory drugs alleviate l-dopa-induced dyskinesia in a rat model of Parkinson's disease
- PMID: 31335998
- PMCID: PMC11719776
- DOI: 10.1002/mds.27799
Immunomodulatory drugs alleviate l-dopa-induced dyskinesia in a rat model of Parkinson's disease
Erratum in
-
Corrigendum.Mov Disord. 2021 Jan;36(1):275. doi: 10.1002/mds.28418. Mov Disord. 2021. PMID: 33492794 No abstract available.
Abstract
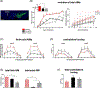
Background: Thalidomide and closely related analogues are used clinically for their immunomodulatory and antiangiogenic properties mediated by the inhibition of the proinflammatory cytokine tumor necrosis factor α. Neuroinflammation and angiogenesis contribute to classical neuronal mechanisms underpinning the pathophysiology of l-dopa-induced dyskinesia, a motor complication associated with l-dopa therapy in Parkinson's disease. The efficacy of thalidomide and the more potent derivative 3,6'-dithiothalidomide on dyskinesia was tested in the 6-hydroxydopamine Parkinson's disease model.
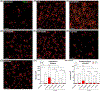
Methods: Three weeks after 6-hydroxydopamine infusion, rats received 10 days of treatment with l-dopa plus benserazide (6 mg/kg each) and thalidomide (70 mg/kg) or 3,6'-dithiothalidomide (56 mg/kg), and dyskinesia and contralateral turning were recorded daily. Rats were euthanized 1 hour after the last l-dopa injection, and levels of tumor necrosis factor-α, interleukin-10, OX-42, vimentin, and vascular endothelial growth factor immunoreactivity were measured in their striatum and substantia nigra reticulata to evaluate neuroinflammation and angiogenesis. Striatal levels of GLUR1 were measured as a l-dopa-induced postsynaptic change that is under tumor necrosis factor-α control.
Results: Thalidomide and 3,6'-dithiothalidomide significantly attenuated the severity of l-dopa-induced dyskinesia while not affecting contralateral turning. Moreover, both compounds inhibited the l-dopa-induced microgliosis and excessive tumor necrosis factor-α in the striatum and substantia nigra reticulata, while restoring physiological levels of the anti-inflammatory cytokine interleukin-10. l-Dopa-induced angiogenesis was inhibited in both basal ganglia nuclei, and l-dopa-induced GLUR1 overexpression in the dorsolateral striatum was restored to normal levels.
Conclusions: These data suggest that decreasing tumor necrosis factor-α levels may be useful to reduce the appearance of dyskinesia, and thalidomide, and more potent derivatives may provide an effective therapeutic approach to dyskinesia. © 2019 International Parkinson and Movement Disorder Society.
Keywords: l-dopa; 3,6′-dithiothalidomide; dyskinesia; immunomodulation; thalidomide.
© 2019 International Parkinson and Movement Disorder Society.
Conflict of interest statement
Authors do not have any financial disclosure/conflicts of interest concerning the research related in this article. No authors have received any funding from any institution, including personal relationships, interests, grants, employment, affiliations, patents, inventions, honoraria, consultancies, royalties, stock options/ownership, or expert testimony for the last 12 months.
Figures



References
-
- Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 2019;18(1):41–58. - PubMed
-
- Franks ME, Macpherson GR, Figg WD. Thalidomide. Lancet 2004; 363(9423):1802–1811. - PubMed
-
- Millrine D, Kishimoto T. A Brighter Side to Thalidomide: Its Potential Use in Immunological Disorders. Trends Mol Med 2017;23(4):348–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

