CS-PEI/Beclin-siRNA Downregulate Multidrug Resistance Proteins and Increase Paclitaxel Therapeutic Efficacy against NSCLC
- PMID: 31336235
- PMCID: PMC6656922
- DOI: 10.1016/j.omtn.2019.06.017
CS-PEI/Beclin-siRNA Downregulate Multidrug Resistance Proteins and Increase Paclitaxel Therapeutic Efficacy against NSCLC
Abstract
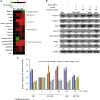
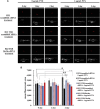
Paclitaxel (PTX) is a widely used chemotherapy drug; however, frequent use causes multidrug resistance (MDR), which limits the utility of PTX against advanced non-small-cell lung cancer (NSCLC). PTX-resistant subline (NCI-H23-TXR) was established in vitro by exposing NCI-H23 cells to gradually increased concentrations of PTX in culture medium. Distinct Beclin expression of autophagy level was observed between resistant NCI-H23-TXR and parental NCI-H23 cells. Beclin-small interfering RNA (siRNA) was selected to restore sensitivity of PTX against NCI-H23-TXR. Chondroitin sulfate-polyethylenimine (CS-PEI) was constructed for delivery and protection of Beclin-siRNA. To delineate the underlying molecular mechanism of Beclin knockdown, we analyzed different MDR expression proteins of two cells using western blot, and the corresponding genes were confirmed by real-time PCR. Compared with NCI-H23, NCI-H23-TXR had higher expression levels in P-glycoprotein (P-gp) and multidrug resistance protein 7 (ABCC10). Knockdown of Beclin simultaneously inhibited P-gp and ABCC10, and renewed the sensitivity of PTX against NCI-H23-TXR. Research on zebrafish embryos revealed that tumor sizes decreased in NCI-H23 tumor xenografts but remained intact in NCI-H23-TXR tumor xenografts as zebrafish were treated with 1 μg/mL PTX. In contrast, the tumor sizes decreased in NCI-H23-TXR tumor xenografts with zebrafish pre-transfected with CS-PEI/Beclin-siRNA followed by the same treatment of PTX. The role of autophagy was associated with MDR development. This study paves the way for a new avenue of PTX in MDR-related lung cancer therapy using CS-PEI as a gene delivery carrier.
Keywords: Beclin-siRNA; MDR; PTX; autophagy; multidrug resistance; non-viral gene delivery vector; paclitaxel.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Yeh J.J., Hsu W.H., Wang J.J., Ho S.T., Kao A. Predicting chemotherapy response to paclitaxel-based therapy in advanced non-small-cell lung cancer with P-glycoprotein expression. Respiration. 2003;70:32–35. - PubMed
-
- Kim Y.D., Park T.E., Singh B., Maharjan S., Choi Y.J., Choung P.H., Arote R.B., Cho C.S. Nanoparticle-mediated delivery of siRNA for effective lung cancer therapy. Nanomedicine (Lond.) 2015;10:1165–1188. - PubMed
-
- Georgiadis M.S., Russell E.K., Gazdar A.F., Johnson B.E. Paclitaxel cytotoxicity against human lung cancer cell lines increases with prolonged exposure durations. Clin. Cancer Res. 1997;3:449–454. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

