Tumor Angiogenic Inhibition Triggered Necrosis (TAITN) in Oral Cancer
- PMID: 31336612
- PMCID: PMC6678844
- DOI: 10.3390/cells8070761
Tumor Angiogenic Inhibition Triggered Necrosis (TAITN) in Oral Cancer
Abstract
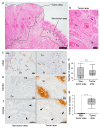
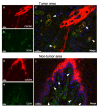
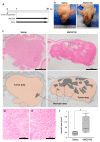
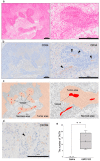
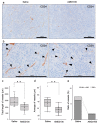
CXCR4 is a chemokine receptor crucial in tumor progression, although the angiogenic role of CXCR4 in oral squamous cell carcinoma (OSCC) has not been investigated. Here we show that CXCR4 is crucial for tumor angiogenesis, thereby supporting tumor survival in OSCC. Immunohistochemistry on human clinical specimens revealed that CXCR4 and a tumor vasculature marker CD34 were co-distributed in tumor vessels in human OSCC specimens. To uncover the effects of CXCR4 inhibition, we treated the OSCC-xenografted mice with AMD3100, so-called plerixafor, an antagonist of CXCR4. Notably, we found a unique pathophysiological structure defined as tumor angiogenic inhibition triggered necrosis (TAITN), which was induced by the CXCR4 antagonism. Treatment with AMD3100 increased necrotic areas with the induction of hypoxia-inducible factor-1α in the xenografted tumors, suggesting that AMD3100-induced TAITN was involved in hypoxia and ischemia. Taken together, we demonstrated that CXCR4 plays a crucial role in tumor angiogenesis required for OSCC progression, whereas TAITN induced by CXCR4 antagonism could be an effective anti-angiogenic therapeutic strategy in OSCC treatment.
Keywords: CXCR4 antagonist; hypoxia; oral squamous cell carcinoma; tumor angiogenic inhibition triggered necrosis (TAITN); tumor blood vessel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hauser A.E., Debes G.F., Arce S., Cassese G., Hamann A., Radbruch A., Manz R.A. Chemotactic Responsiveness Toward Ligands for CXCR3 and CXCR4 Is Regulated on Plasma Blasts During the Time Course of a Memory Immune Response. J. Immunol. 2002;169:1277–1282. doi: 10.4049/jimmunol.169.3.1277. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

