CD56 Homodimerization and Participation in Anti-Tumor Immune Effector Cell Functioning: A Role for Interleukin-15
- PMID: 31336622
- PMCID: PMC6678613
- DOI: 10.3390/cancers11071029
CD56 Homodimerization and Participation in Anti-Tumor Immune Effector Cell Functioning: A Role for Interleukin-15
Abstract
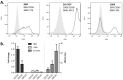
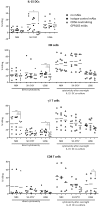
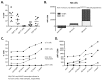
A particularly interesting marker to identify anti-tumor immune cells is the neural cell adhesion molecule (NCAM), also known as cluster of differentiation (CD)56. Namely, hematopoietic expression of CD56 seems to be confined to powerful effector immune cells. Here, we sought to elucidate its role on various killer immune cells. First, we identified the high motility NCAM-120 molecule to be the main isoform expressed by immune cells. Next, through neutralization of surface CD56, we were able to (1) demonstrate the direct involvement of CD56 in tumor cell lysis exerted by CD56-expressing killer cells, such as natural killer cells, gamma delta (γδ) T cells, and interleukin (IL)-15-cultured dendritic cells (DCs), and (2) reveal a putative crosstalk mechanism between IL-15 DCs and CD8 T cells, suggesting CD56 as a co-stimulatory molecule in their cell-to-cell contact. Moreover, by means of a proximity ligation assay, we visualized the CD56 homophilic interaction among cancer cells and between immune cells and cancer cells. Finally, by blocking the mitogen-activated protein kinase (MAPK) pathway and the phosphoinositide 3-kinase (PI3K)-Akt pathway, we showed that IL-15 stimulation directly led to CD56 upregulation. In conclusion, these results underscore the previously neglected importance of CD56 expression on immune cells, benefiting current and future immune therapeutic options.
Keywords: CD56 homodimers; NCAM-120; common gamma-chain family; interleukin-15 signaling; tumor cell eradication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Sangiolo D., Martinuzzi E., Todorovic M., Vitaggio K., Vallario A., Jordaney N., Carnevale-Schianca F., Capaldi A., Geuna M., Casorzo L., et al. Alloreactivity and anti-tumor activity segregate within two distinct subsets of cytokine-induced killer (CIK) cells: Implications for their infusion across major HLA barriers. Int. Immunol. 2008;20:841–848. doi: 10.1093/intimm/dxn042. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

