Exploring the Reservoir Hosts of Tick-Borne Encephalitis Virus
- PMID: 31336624
- PMCID: PMC6669706
- DOI: 10.3390/v11070669
Exploring the Reservoir Hosts of Tick-Borne Encephalitis Virus
Abstract
Tick-borne encephalitis virus (TBEV) is an important arbovirus, which is found across large parts of Eurasia and is considered to be a major health risk for humans. Like any other arbovirus, TBEV relies on complex interactions between vectors, reservoir hosts, and the environment for successful virus circulation. Hard ticks are the vectors for TBEV, transmitting the virus to a variety of animals. The importance of these animals in the lifecycle of TBEV is still up for debate. Large woodland animals seem to have a positive influence on virus circulation by providing a food source for adult ticks; birds are suspected to play a role in virus distribution. Bank voles and yellow-necked mice are often referred to as classical virus reservoirs, but this statement lacks strong evidence supporting their highlighted role. Other small mammals (e.g., insectivores) may also play a crucial role in virus transmission, not to mention the absence of any suspected reservoir host for non-European endemic regions. Theories highlighting the importance of the co-feeding transmission route go as far as naming ticks themselves as the true reservoir for TBEV, and mammalian hosts as a mere bridge for transmission. A deeper insight into the virus reservoir could lead to a better understanding of the development of endemic regions. The spatial distribution of TBEV is constricted to certain areas, forming natural foci that can be restricted to sizes of merely 500 square meters. The limiting factors for their occurrence are largely unknown, but a possible influence of reservoir hosts on the distribution pattern of TBE is discussed. This review aims to give an overview of the multiple factors influencing the TBEV transmission cycle, focusing on the role of virus reservoirs, and highlights the questions that are waiting to be further explored.
Keywords: reservoir; rodent; tick-borne encephalitis; ticks; transmission.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

 ), but can be found in a variety of animals (
), but can be found in a variety of animals ( ). Additionally, humans can become infected by consuming unpasteurized dairy products originating from viremic animals (6). Infected birds are suspected to be a vector for virus passage to new endemic foci, although a spatial restriction seems likely (5). TBEV is distributed within the tick population mainly through trans-stadial (1) transmission, and occasionally through trans-ovarial (2) transmission. For successful virus circulation, the virus needs to be spread within the tick population. This is achieved through naïve ticks consuming their blood meal on viremic host animals, as well as through co-feeding (4).
). Additionally, humans can become infected by consuming unpasteurized dairy products originating from viremic animals (6). Infected birds are suspected to be a vector for virus passage to new endemic foci, although a spatial restriction seems likely (5). TBEV is distributed within the tick population mainly through trans-stadial (1) transmission, and occasionally through trans-ovarial (2) transmission. For successful virus circulation, the virus needs to be spread within the tick population. This is achieved through naïve ticks consuming their blood meal on viremic host animals, as well as through co-feeding (4).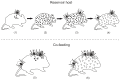
 ) to the animal host (1), leading to viremia (2). Naïve ticks acquire TBEV by consuming the blood of a viremic host (3). As soon as viremia comes to an end, this route of transmission is blocked by circulating antibodies (
) to the animal host (1), leading to viremia (2). Naïve ticks acquire TBEV by consuming the blood of a viremic host (3). As soon as viremia comes to an end, this route of transmission is blocked by circulating antibodies ( ) (4). Co-feeding enables ticks to pass TBEV among themselves without the need for a viremic host. When naïve ticks feed in close proximity with an infected tick, the animal host acts as a transmission bridge (5). This can take place even when the host has antibodies against TBEV (6).
) (4). Co-feeding enables ticks to pass TBEV among themselves without the need for a viremic host. When naïve ticks feed in close proximity with an infected tick, the animal host acts as a transmission bridge (5). This can take place even when the host has antibodies against TBEV (6).References
-
- Süss J. Tick-borne encephalitis in Europe and beyond—The epidemiological situation as of 2007. Euro Surveill. 2008;13:717–727. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

