Synthesis of the Core Framework of the Cornexistins by Intramolecular Nozaki-Hiyama-Kishi Coupling
- PMID: 31336669
- PMCID: PMC6680490
- DOI: 10.3390/molecules24142654
Synthesis of the Core Framework of the Cornexistins by Intramolecular Nozaki-Hiyama-Kishi Coupling
Abstract
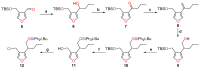
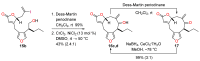
A new and direct approach to the construction of the core framework of the herbicidal natural products cornexistin and hydroxycornexistin has been developed. Formation of the nine-membered carbocycle found in the natural products has been accomplished by an intramolecular Nozaki-Hiyama-Kishi reaction between a vinylic iodide and an aldehyde. Good yields of carbocyclic products were obtained from the reaction, but diastereomeric mixtures of allylic alcohols were produced. The cyclisation reaction was successful irrespective of the relative configuration of the stereogenic centres in the cyclisation precursor.
Keywords: Nozaki-Hiyama-Kishi reaction; cornexistins; cyclisation; herbicide; natural product; nine-membered carbocycle; nonadride.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Haneishi T., Nakajima M., Koi K., Furuya K., Iwado S., Sato S. Anhydride Herbicides. 4897104. U.S. Patent. 1990
-
- Haneishi T., Nakajima M., Koi K., Furuya K., Iwado S., Sata S. Herbicide, Its Preparation and Use. 4990178. U.S. Patent. 1991
-
- Fields S.C., Gerwick B.C., Mireles-Lo L. Hydroxycornexistin. 5424278. U.S. Patent. 1995
-
- Fields S.C., Mireles-Lo L., Gerwick B.C. Hydroxycornexistin: A New Phytotoxin from Paecilomyces Variotii. J. Nat. Prod. 1996;59:698–700. doi: 10.1021/np960205e. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

