Flavopereirine Suppresses the Growth of Colorectal Cancer Cells through P53 Signaling Dependence
- PMID: 31336690
- PMCID: PMC6678721
- DOI: 10.3390/cancers11071034
Flavopereirine Suppresses the Growth of Colorectal Cancer Cells through P53 Signaling Dependence
Abstract
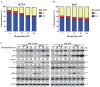
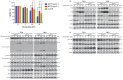
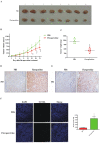
Colorectal cancer (CRC) is a significant cause of morbidity and mortality worldwide. The outcome of CRC patients remains poor. Thus, a new strategy for CRC treatment is urgently needed. Flavopereirine is a β-carboline alkaloid extracted from Geissospermum vellosii, which can reduce the viability of various cancer cells through an unknown mode of action. The aim of the present study was to investigate the functional mechanism and therapeutic potential of flavopereirine on CRC cells in vitro and in vivo. Our data showed that flavopereirine significantly lowered cellular viability, caused intrinsic and extrinsic apoptosis, and induced G2/M-phase cell cycle arrest in CRC cells. Flavopereirine downregulated Janus kinases-signal transducers and activators of transcription (JAKs-STATs) and cellular myelocytomatosis (c-Myc) signaling in CRC cells. In contrast, the enforced expressions of constitutive active STAT3 and c-Myc could not restore flavopereirine-induced viability reduction. Moreover, flavopereirine enhanced P53 expression and phosphorylation in CRC cells. CRC cells with P53 knockout or loss-of-function mutation significantly diminished flavopereirine-mediated viability reduction, indicating that P53 activity plays a major role in flavopereirine-mediated CRC cell growth suppression. Flavopereirine also significantly repressed CRC cell xenograft growth in vivo by upregulating P53 and P21 and inducing apoptosis. In conclusion, flavopereirine-mediated growth suppression in CRC cells depended on the P53-P21, but not the JAKs-STATs-c-Myc signaling pathway. The present study suggests that flavopereirine may be efficacious in the clinical treatment of CRC harboring functional P53 signaling.
Keywords: Flavopereirine; P53; colorectal cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Karapetis C.S., Khambata-Ford S., Jonker D.J., O’Callaghan C.J., Tu D., Tebbutt N.C., Simes R.J., Chalchal H., Shapiro J.D., Robitaille S., et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

