Tebentafusp: T Cell Redirection for the Treatment of Metastatic Uveal Melanoma
- PMID: 31336704
- PMCID: PMC6679206
- DOI: 10.3390/cancers11070971
Tebentafusp: T Cell Redirection for the Treatment of Metastatic Uveal Melanoma
Abstract
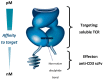
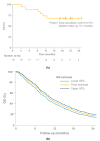
Metastatic disease from uveal melanoma occurs in almost 50% of patients suffering from this ocular tumour, with median survival from development of symptoms being around 1 year. In contrast to cutaneous melanoma, kinase inhibitors and immune checkpoint inhibitors are usually ineffective in patients with metastatic uveal melanoma. Tebentafusp is a novel form of immunotherapy based on the immune-mobilising monoclonal T cell receptor against cancer (ImmTAC) platform, which comprises a soluble T cell receptor that is fused to an anti-CD3 single-chain variable fragment. The T cell receptor domain of tebentafusp targets cells present a human leukocyte antigen-A*02:01 complexed with a peptide derived from the melanoma-associated antigen gp100, which is expressed strongly by melanoma cells, weakly by normal melanocytes and minimally by other tissues. The anti-CD3 domain recruits CD3+ T cells (and, indirectly, other immune cells), redirecting these to the melanoma cells. The most common adverse events with tebentafusp are manageable and usually transient. Early survival data in patients with metastatic uveal melanoma are promising when considered alongside historical data. Based on these encouraging results, a randomised study comparing tebentafusp to investigator's choice of therapy in metastatic uveal melanoma is ongoing.
Keywords: ImmTAC platform; T cell; T cell receptor; anti-CD3 bispecific; clinical data; immunotherapy; metastatic uveal melanoma; preclinical data; tebentafusp.
Conflict of interest statement
Bertil Damato has received consulting fees from Immunocore. Rich Carvajal has received consulting fees and research funding to institution from Immunocore. Howard Goodall and Joseph Dukes are employees of Immunocore.
Figures



References
-
- Sato T., Nathan P.D., Hernandez-Aya L., Sacco J., Orloff M., Engler F., Little N., Hulstine A., Coughlin C., Carvajal R.D. Redirected T cell mediated lysis in patients with metastatic uveal melanoma with gp100-directed TCR IMCgp100: Overall survival findings; Proceedings of the American Society of Clinical Oncology Annual Meeting; Chicago, IL, USA. 1–5 June 2018; Poster 9521.
-
- Middleton M., Steven N., Evans J., Infante J., Sznol M. Safety, pharmacokinetics and efficacy of IMCgp100, a first-in-class soluble TCR anti-CD3 bispecific T cell redirector with solid tumour activity: Results from the first in human study in melanoma; Proceedings of the American Society of Clinical Oncology Annual Meeting; Chicago, IL, USA. 3–7 June 2016; Poster 3016.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

