Antenatal Influenza A-Specific IgA, IgM, and IgG Antibodies in Mother's Own Breast Milk and Donor Breast Milk, and Gastric Contents and Stools from Preterm Infants
- PMID: 31336756
- PMCID: PMC6682892
- DOI: 10.3390/nu11071567
Antenatal Influenza A-Specific IgA, IgM, and IgG Antibodies in Mother's Own Breast Milk and Donor Breast Milk, and Gastric Contents and Stools from Preterm Infants
Abstract
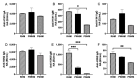
Antenatal milk anti-influenza antibodies may provide additional protection to newborns until they are able to produce their own antibodies. To evaluate the relative abundance of milk, we studied the antibodies specific to influenza A in feeds and gastric contents and stools from preterm infants fed mother's own breast milk (MBM) and donor breast milk (DBM). Feed (MBM or DBM) and gastric contents (MBM or DBM at 1 h post-ingestion) and stool samples (MBM/DBM at 24 h post-ingestion) were collected, respectively, from 20 preterm (26-36 weeks gestational age) mother-infant pairs at 8-9 days and 21-22 days of postnatal age. Samples were analyzed via ELISA for anti-H1N1 hemagglutinin (anti-H1N1 HA) and anti-H3N2 neuraminidase (anti-H3N2 NA) specificity across immunoglobulin A (IgA), immunoglobulin M (IgM), and immunoglobulin G (IgG) isotypes. The relative abundance of influenza A-specific IgA in feeds and gastric contents were higher in MBM than DBM at 8-9 days of postnatal age but did not differ at 21-22 days. Anti-influenza A-specific IgM was higher in MBM than in DBM at both postnatal times in feed and gastric samples. At both postnatal times, anti-influenza A-specific IgG was higher in MBM than DBM but did not differ in gastric contents. Gastric digestion reduced anti-H3N2 NA IgG from MBM at 21-22 days and from DBM at 8-9 days of lactation, whereas other anti-influenza A antibodies were not digested at either postnatal times. Supplementation of anti-influenza A-specific antibodies in DBM may help reduce the risk of influenza virus infection. However, the effective antibody dose required to induce a significant protective effect remains unknown.
Keywords: flu vaccine; human milk; lactation; maternal immunoglobulins; passive immunization; prematurity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Salam R.A., Das J.K., Soeandy C.D., Lassi Z.S., Bhutta Z.A. Impact of Haemophilus influenzae type B (Hib) and viral influenza vaccinations in pregnancy for improving maternal, neonatal and infant health outcomes. Cochrane Datab. Syst. Rev. 2015;6:1–41. doi: 10.1002/14651858.CD009982.pub2. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

