The Effects of Imitation Dynamics on Vaccination Behaviours in SIR-Network Model
- PMID: 31336761
- PMCID: PMC6678199
- DOI: 10.3390/ijerph16142477
The Effects of Imitation Dynamics on Vaccination Behaviours in SIR-Network Model
Abstract
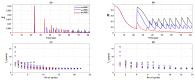
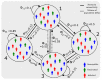
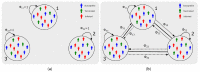
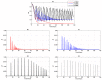
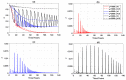
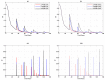
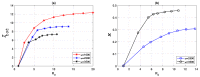
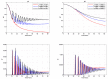
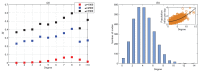
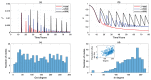
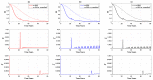
We present a series of SIR-network models, extended with a game-theoretic treatment of imitation dynamics which result from regular population mobility across residential and work areas and the ensuing interactions. Each considered SIR-network model captures a class of vaccination behaviours influenced by epidemic characteristics, interaction topology, and imitation dynamics. Our focus is the resultant vaccination coverage, produced under voluntary vaccination schemes, in response to these varying factors. Using the next generation matrix method, we analytically derive and compare expressions for the basic reproduction number R 0 for the proposed SIR-network models. Furthermore, we simulate the epidemic dynamics over time for the considered models, and show that if individuals are sufficiently responsive towards the changes in the disease prevalence, then the more expansive travelling patterns encourage convergence to the endemic, mixed equilibria. On the contrary, if individuals are insensitive to changes in the disease prevalence, we find that they tend to remain unvaccinated. Our results concur with earlier studies in showing that residents from highly connected residential areas are more likely to get vaccinated. We also show that the existence of the individuals committed to receiving vaccination reduces R 0 and delays the disease prevalence, and thus is essential to containing epidemics.
Keywords: Erdös-Rényi random networks; SIR model; epidemic modelling; greater Sydney commuting network; herd immunity; strategy imitation; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- World Heath Organization . Basic Epidemiology. World Heath Organization; Geneva, Switzerland: 2006. pp. 125–127.
-
- Wang Z., Bauch C., Bhattacharyya S., d’Onofrio A., Manfredi P., Perc M.P.N., Salathè M., Zhao D. Statistical physics of vaccination. Phys. Rep. 2016;664:1–113. doi: 10.1016/j.physrep.2016.10.006. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

