Fabrication of Human Keratinocyte Cell Clusters for Skin Graft Applications by Templating Water-in-Water Pickering Emulsions
- PMID: 31336810
- PMCID: PMC6784416
- DOI: 10.3390/biomimetics4030050
Fabrication of Human Keratinocyte Cell Clusters for Skin Graft Applications by Templating Water-in-Water Pickering Emulsions
Abstract
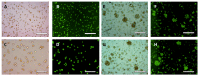
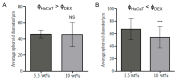
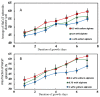
Most current methods for the preparation of tissue spheroids require complex materials, involve tedious physical steps and are generally not scalable. We report a novel alternative, which is both inexpensive and up-scalable, to produce large quantities of viable human keratinocyte cell clusters (clusteroids). The method is based on a two-phase aqueous system of incompatible polymers forming a stable water-in-water (w/w) emulsion, which enabled us to rapidly fabricate cell clusteroids from HaCaT cells. We used w/w Pickering emulsion from aqueous solutions of the polymers dextran (DEX) and polyethylene oxide (PEO) and a particle stabilizer based on whey protein (WP). The HaCaT cells clearly preferred to distribute into the DEX-rich phase and this property was utilized to encapsulate them in the water-in-water (DEX-in-PEO) emulsion drops then osmotically shrank to compress them into clusters. Prepared formulations of HaCaT keratinocyte clusteroids in alginate hydrogel were grown where the cells percolated to mimic 3D tissue. The HaCaT cell clusteroids grew faster in the alginate film compared to the individual cells formulated in the same matrix. This methodology could potentially be utilised in biomedical applications.
Keywords: DEX; HaCaT; PEO; Pickering emulsions; alginate; hydrogels; keratinocyte; spheroids; tissue engineering; water-in-water emulsions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

