Nitropyridine-1-Oxides as Excellent π-Hole Donors: Interplay between σ-Hole (Halogen, Hydrogen, Triel, and Coordination Bonds) and π-Hole Interactions
- PMID: 31336936
- PMCID: PMC6678756
- DOI: 10.3390/ijms20143440
Nitropyridine-1-Oxides as Excellent π-Hole Donors: Interplay between σ-Hole (Halogen, Hydrogen, Triel, and Coordination Bonds) and π-Hole Interactions
Abstract
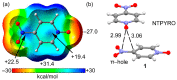
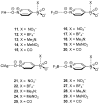
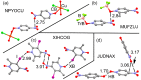
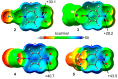
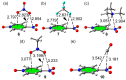
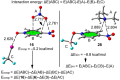
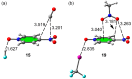
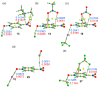
In this manuscript, we use the primary source of geometrical information, i.e., Cambridge Structural Database (CSD), combined with density functional theory (DFT) calculations (PBE0-D3/def2-TZVP level of theory) to demonstrate the relevance of π-hole interactions in para-nitro substituted pyridine-1-oxides. More importantly, we show that the molecular electrostatic potential (MEP) value above and below the π-hole of the nitro group is largely influenced by the participation of the N-oxide group in several interactions like hydrogen-bonding (HB) halogen-bonding (XB), triel bonding (TrB), and finally, coordination-bonding (CB) (N+-O- coordinated to a transition metal). The CSD search discloses that p-nitro-pyridine-1-oxide derivatives have a strong propensity to participate in π-hole interactions via the nitro group and, concurrently, N-oxide group participates in a series of interactions as electron donor. Remarkably, the DFT calculations show from strong to moderate cooperativity effects between π-hole and HB/XB/TrB/CB interactions (σ-bonding). The synergistic effects between π-hole and σ-hole bonding interactions are studied in terms of cooperativity energies, using MEP surface analysis and the Bader's quantum theory of atoms in molecules (QTAIM).
Keywords: CSD analysis; cooperativity; supramolecular chemistry; π-hole interactions; σ-hole interactions.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Theoretical ab Initio Study on Cooperativity Effects between Nitro π-hole and Halogen Bonding Interactions.Chemphyschem. 2019 May 3;20(9):1135-1144. doi: 10.1002/cphc.201900142. Epub 2019 Apr 10. Chemphyschem. 2019. PMID: 30884031
-
Asymmetric bifurcated halogen bonds.Phys Chem Chem Phys. 2015 Mar 7;17(9):6440-50. doi: 10.1039/c4cp05532b. Phys Chem Chem Phys. 2015. PMID: 25656525
-
Substituent Effects in π-Hole Regium Bonding Interactions Between Au(p-X-Py)2 Complexes and Lewis Bases: An ab initio Study.Chemphyschem. 2022 Apr 20;23(8):e202200010. doi: 10.1002/cphc.202200010. Epub 2022 Mar 8. Chemphyschem. 2022. PMID: 35191571
-
Metal Coordination Enhances Chalcogen Bonds: CSD Survey and Theoretical Calculations.Int J Mol Sci. 2022 Apr 10;23(8):4188. doi: 10.3390/ijms23084188. Int J Mol Sci. 2022. PMID: 35457005 Free PMC article. Review.
-
Noncovalent interactions in proteins and nucleic acids: beyond hydrogen bonding and π-stacking.Chem Soc Rev. 2022 Jun 6;51(11):4261-4286. doi: 10.1039/d2cs00133k. Chem Soc Rev. 2022. PMID: 35560317 Review.
Cited by
-
Host-Guest Complexes.Int J Mol Sci. 2022 Dec 12;23(24):15730. doi: 10.3390/ijms232415730. Int J Mol Sci. 2022. PMID: 36555372 Free PMC article.
-
The Physical Chemistry and Chemical Physics (PCCP) Section of the International Journal of Molecular Sciences in Its Publications: The First 300 Thematic Articles in the First 3 Years.Int J Mol Sci. 2021 Dec 27;23(1):241. doi: 10.3390/ijms23010241. Int J Mol Sci. 2021. PMID: 35008667 Free PMC article.
-
Molecular structure and electron distribution of 4-nitropyridine N-oxide: Experimental and theoretical study of substituent effects.J Mol Struct. 2020 Oct 5;1217:128476. doi: 10.1016/j.molstruc.2020.128476. Epub 2020 May 17. J Mol Struct. 2020. PMID: 32427177 Free PMC article.
-
Comparative Structural Study of Three Tetrahalophthalic Anhydrides: Recognition of X···O(anhydride) Halogen Bond and πh···O(anhydride) Interaction.Molecules. 2021 May 23;26(11):3119. doi: 10.3390/molecules26113119. Molecules. 2021. PMID: 34071107 Free PMC article.
-
Complexes of Zinc-Coordinated Heteroaromatic N-Oxides with Pyrene: Lewis Acid Effects on the Multicenter Donor-Acceptor Bonding.Molecules. 2024 Jul 13;29(14):3305. doi: 10.3390/molecules29143305. Molecules. 2024. PMID: 39064884 Free PMC article.
References
-
- Lehn J.M. Supramolecular Chemistry Concepts and Perspectives. Wiley-VCH; Weinheim, Germany: 1995.
-
- Steed J.W., Atwood J.L. Supramolecular Chemistry. Wiley; Chichester, UK: 2000.
-
- Gilli G., Gilli P. The Nature of the Hydrogen Bond. Oxford University Press; Oxford, UK: 2009.
-
- Desiraju G.R., Steiner T. The Weak Hydrogen Bond in Structural Chemistry and Biology. International Union of Crystal; Oxford, UK: New York, NY, USA: 1999.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

