Three New Mutations and Mild, Asymmetrical Phenotype in the Highly Distinctive LAMM Syndrome: A Report of Eight Further Cases
- PMID: 31336982
- PMCID: PMC6678228
- DOI: 10.3390/genes10070529
Three New Mutations and Mild, Asymmetrical Phenotype in the Highly Distinctive LAMM Syndrome: A Report of Eight Further Cases
Abstract
Labyrinthine aplasia, microtia, and microdontia (LAMM) is an autosomal recessive condition causing profound congenital deafness, complete absence of inner ear structures (usually Michel's aplasia), microtia (usually type 1) and microdontia. To date, several families have been described with this condition and a number of mutations has been reported. We report on eight further cases of LAMM syndrome including three novel mutations, c. 173T>C p.L58P; c. 284G>A p.(Arg95Gln) and c.325_327delinsA p.(Glu109Thrfs*18). Congenital deafness was the primary presenting feature in all affected individuals and consanguinity in all but two families. We compare the features in our patients to those previously reported in LAMM, and describe a milder, asymmetrical phenotype associated with FGF3 mutations.
Keywords: FGF3; LAMM syndrome; and microdontia; congenital deafness; external ear abnormalities; labyrinthine aplasia; microtia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
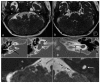


References
-
- Latchman K., Tekin M. Labyrinthine Aplasia, Microtia, and Microdontia (LAMM) Syndrome and FGF3 Mutations. Nova Science Publishers, Inc.; Hauppauge, NY, USA: 2013.
-
- Tekin M., Hişmi B.O., Fitoz S., Ozdag H., Cengiz F.B., Sırmacı A., Aslan I., Inceoglu B., Yüksel-Konuk E.B., Yılmaz S.T., et al. Homozygous Mutations in Fibroblast Growth Factor 3 Are Associated with a New Form of Syndromic Deafness Characterized by Inner Ear Agenesis, Microtia, and Microdontia. Am. J. Hum. Genet. 2007;80:338–344. doi: 10.1086/510920. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

