Development and Characterization of Monoclonal Antibodies to Botulinum Neurotoxin Type E
- PMID: 31337022
- PMCID: PMC6669634
- DOI: 10.3390/toxins11070407
Development and Characterization of Monoclonal Antibodies to Botulinum Neurotoxin Type E
Abstract
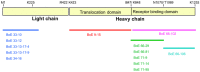
Botulism is a devastating disease caused by botulinum neurotoxins (BoNTs) secreted primarily by Clostridium botulinum. Mouse bioassays without co-inoculation with antibodies are the standard method for the detection of BoNTs, but are not capable of distinguishing between the different serotypes (A-G). Most foodborne intoxications are caused by serotypes BoNT/A and BoNT/B. BoNT/E outbreaks are most often observed in northern coastal regions and are associated with eating contaminated marine animals and other fishery products. Sandwich enzyme-linked immunosorbent assays (ELISAs) were developed for the detection of BoNT/E3. Monoclonal antibodies (mAbs) were generated against BoNT/E3 by immunizing with recombinant peptide fragments of the light and heavy chains of BoNT/E3. In all, 12 mAbs where characterized for binding to both the recombinant peptides and holotoxin, as well as their performance in Western blots and sandwich ELISAs. The most sensitive sandwich assay, using different mAbs for capture and detection, exhibited a limit of detection of 0.2 ng/ml in standard buffer matrix and 10 ng/mL in fish product matrices. By employing two different mAbs for capture and detection, a more standardized sandwich assay was constructed. Development of sensitive and selective mAbs to BoNT/E would help in the initial screening of potential food contamination, speeding diagnosis and reducing use of laboratory animals.
Keywords: BoNT/E; ELISA; botulinum neurotoxin serotype E; immunoassay; monoclonal antibodies.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- CDC . Botulism Annual Summary, 2016. US Department of Health and Human Services; Atlanta, Georgia: 2017. [(accessed on 1 April 2019)]. Available online: https://www.cdc.gov/botulism/pdf/Botulism-2016-SUMMARY-508.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

