Physical and Functional Analysis of Viral RNA Genomes by SHAPE
- PMID: 31337286
- PMCID: PMC6768749
- DOI: 10.1146/annurev-virology-092917-043315
Physical and Functional Analysis of Viral RNA Genomes by SHAPE
Abstract
RNA viruses encode the information required to usurp cellular metabolism and gene regulation and to enable their own replication in two ways: in the linear sequence of their RNA genomes and in higher-order structures that form when the genomic RNA strand folds back on itself. Application of high-resolution SHAPE (selective 2'-hydroxyl acylation analyzed by primer extension) structure probing to viral RNA genomes has identified numerous new regulatory elements, defined new principles by which viral RNAs interact with the cellular host and evade host immune responses, and revealed relationships between virus evolution and RNA structure. This review summarizes our current understanding of genome structure-function interrelationships for RNA viruses, as informed by SHAPE structure probing, and outlines opportunities for future studies.
Keywords: RNA structure; RNA viruses; SHAPE; chemical probing; functional validation.
Figures
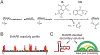

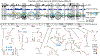
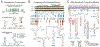

References
-
- Brion P, Westhof E. 1997. Hierarchy and Dynamics of RNA Folding. Annu. Rev. Biophys. Biomol. Struct 26(1):113–37 - PubMed
-
- Batey RT, Rambo RP, Doudna JA. 1999. Tertiary motifs in RNA structure and folding. Angew. Chem. - Int. Ed 38(16):2326–43 - PubMed
-
- Butcher SE, Pyle AM. 2011. The molecular interactions that stabilize RNA tertiary structure: RNA motifs, patterns, and networks. Acc. Chem. Res 44(12):1302–11 - PubMed
-
- Desselberger U, Racaniello VR, Zazra JJ, Palese P. 1980. The 3’ and 5’-terminal sequences of influenza A, B and C virus RNA segments are highly conserved and show partial inverted complementarity. Gene 8(3):315–28 - PubMed
-
- Pflug A, Guilligay D, Reich S, Cusack S. 2014. Structure of influenza A polymerase bound to the viral RNA promoter. Nature 516(7531):355–60 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

