Portal Protein: The Orchestrator of Capsid Assembly for the dsDNA Tailed Bacteriophages and Herpesviruses
- PMID: 31337287
- PMCID: PMC6947915
- DOI: 10.1146/annurev-virology-092818-015819
Portal Protein: The Orchestrator of Capsid Assembly for the dsDNA Tailed Bacteriophages and Herpesviruses
Abstract
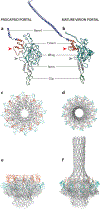
Tailed, double-stranded DNA bacteriophages provide a well-characterized model system for the study of viral assembly, especially for herpesviruses and adenoviruses. A wealth of genetic, structural, and biochemical work has allowed for the development of assembly models and an understanding of the DNA packaging process. The portal complex is an essential player in all aspects of bacteriophage and herpesvirus assembly. Despite having low sequence similarity, portal structures across bacteriophages share the portal fold and maintain a conserved function. Due to their dynamic role, portal proteins are surprisingly plastic, and their conformations change for each stage of assembly. Because the maturation process is dependent on the portal protein, researchers have been working to validate this protein as a potential antiviral drug target. Here we review recent work on the role of portal complexes in capsid assembly, including DNA packaging, as well as portal ring assembly and incorporation and analysis of portal structures.
Keywords: DNA packaging; bacteriophage assembly; portal fold; virus assembly.
Figures



References
-
- Prevelige PE Jr., King J 1993. Assembly of bacteriophage P22: a model for ds-DNA virus assembly. Prog. Med. Virol. 40:206–21 - PubMed
-
- Prevelige PE, Thomas D, King J. 1988. Scaffolding protein regulates the polymerization of P22 coat subunits into icosahedral shells in vitro. J. Mol. Biol. 202:743–57 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

